Photomorphogenesis
I. Introduction
A. Photomorphogenesis: control of plant morphological
development by light.
B. Photoreceptors:
1. Phytochrome: a chromoprotein that absorbs red and far-red
light as well as blue light. There are two major forms of
phytochrome; type 1 from etiolated seedlings and type 2
from green plants.
2. Cryptochrome: an unidentified group of favonoid pigments
that absorb strongly in the blue and long-wave ultraviolet
wavelengths. Named from its importance in cryptogams (non-
flowering plants).
3. UV-B photoreceptor: compounds that absorb ultraviolet light
between 280 and 320 nm.
4. Protochlorophyllide a: the immediate precursor of
chlorophyll a that absorbs red and blue light in order to
make the change to chlorophyll.
C. The most important factor controlling light responses in
photomorphogenesis is the competence to respond or
sensitivity of the receptor cells. Besides the need for an
amplification system like Ca2+ and calmodulin, there are two
stages that are important to the process of
photomorphogenesis:
1. Pattern Specification: developing cells and tissues become
competent to react to light
2. Pattern Realization: the competent cells and tissues
respond to light.
 II. Phytochrome
A. A brief history:
1. Early work on the effect of flowering was done by W.W.
Garner and H.A. Allard in the 1920's. The found that the
duration of light and dark periods controlled flowering in
plants. One of the major research species was cocklebur
with required nights longer than a critical minimum to
flower (i.e. short day plants). It was found that different
wavelengths of light had different abilities to induce
flowering. Red light was the most effective in inducing
flowering.
2. Most of the research that first described phytochrome was
done between 1945 and 1960 at the U.S.D.A. in Beltsville,
Maryland by Harry Borthwick and Sterling Hendricks.
Borthwick and Hendricks worked with seed germination of Grand
Rapids lettuce seeds. They completed an action spectrum for
promotion of germination. There was a peak of seed
germination in the red wavelengths that could be reversed by
treatment with far-red light (wavelengths between 700-800
nm). They found that what ever light treatment was given
last determined whether the seeds germinated. This finding
laid the ground work for the discovery and isolation of
phytochrome.
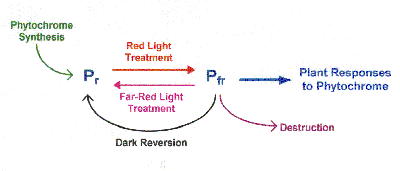
B. Phytochrome and its properties:
1. There are two forms of phytochrome. The Pr form which
absorbs red light is blue in color. When Pr absorbs red
light it is converted to the Pfr form which is olive-green
in color. The Pfr form absorbs far-red light and is
subsequently converted into the Pr form. The Pr form is
considered the active form of phytochrome.
2. Absorption spectra for phytochrome:
a. Angiosperms: maxima in red wavelengths is 666 nm (Pr
absorbing form)
maxima in far-red wavelength is 730 nm (Pfr
absorbing form)
b. Found in angiosperms, gymnosperms, ferns, mosses,
liverworts, and green, red, and brown algae. The protein
moieties are different but the chromophore is the same in
all species. Phytochrome is synthesized in the Pr form.
Phytochrome is found in the cytosol and nucleus but does
not seem to be associated with any particular organelle.
3. Phytochrome is a chromoprotein. The protein portion is a
homodimer consisting of two identical polypeptides of 120
kDa. The chromophore is an open-chain tetrapyrrole
connected to the protein moiety through the sulfur of a
cysteine. When phytochrome absorbs red light there is a
cis-trans isomerization of the chromophore which causes
changes in the protein moiety. The changes in the protein
are responsible for the physiological changes.
4. When the Pr form absorbs red light about 85% of it is
converted to Pfr. Pfr is also converted back to Pr because
it can absorb a small amount of the red light.
5. There are two types of phytochrome (each can exists in the
Pr or Pfr form):
a. Type 1: found in etiolated seedlings
b. Type 2: found in green plants. Pr absorption maxima is
654 nm for red light and 724 nm for far-red light.
Several (at least five) different protein moieties have
been found.
c. Seedlings grown in the dark have much more type 1
phytochrome than type 2 phytochrome. This allows the
seedlings to respond to very low levels of red light.
Upon absorption of red light the majority of type 1
phytochrome is lost. There are three controlling factors
that govern this lose of type 1 phytochrome: (1) mRNA for
type 1 phytochrome is no longer produced, (2) mRNA for
type 1 phytochrome is highly unstable and is therefore
quickly degraded, (3) the type 1 phytochrome itself is
quickly degraded. Phytochrome self regulates the gene for
type 1 phytochrome production. This gene is active in the
dark, but is inactive in the light.
6. Evidence that support phytochrome as the pigment responsible
for photomorphogenesis are: (1) a correlation between the
absorption spectrum of phytochrome and the action spectra
for plant responses, (2) plant responses to red light can
be immediately reversed by exposure to far-red light, (3)
the irradiance level for the plant responses is quite low.
7. f (Psi)=Pfr/Pr + Pfr: the ratio of Pfr to the total amount
of phytochrome of both forms. This value is used to
describe the amount of phytochrome in a plant tissue. In
full sunlight the ratio of red to far-red photons is 1.1-
1.2. Pr absorbs red light more efficiently than Pfr
absorbs far-red light, which means that Pr will be converted
to Pfr quicker than Pfr can convert back to Pr. Therefor,
in sun light there is more Pfr than Pr and the f (Psi) value
is about 0.6.
8. If type 1 phytochrome found in etiolated plants receives a
brief red-light treatment, some of the Pfr formed will
gradually disappear over time. This disappearance of Pfr is
due to: (1) destruction or breakdown of Pfr by attachment to
ubiquitin (a 76 amino acid protein) which targets the Pfr
for degradation by proteases, or (2) dark reversion which
takes several hours. Dark reversion occurs in gymnosperms
and dicots, but not monocots.
9. Type 2 phytochrome does not undergo destruction or dark
reversion because it is more stable.
III. Cryptochrome:
In 1864 Julius vonSachs discovered that blue light caused
phototropism. Later it was found that near ultraviolet (UV)
light called UV-A radiation between 320 and 400 nm was also
involved in many plant developmental processes. The pigment
responsible for the absorption of light at these wavelengths is
called cryptochrome. Cryptochrome is a flavoprotein that is
associated with a cytochrome protein in the plasma membrane. Its
action spectrum maximum is found at 450 nm.
IV. Photomorphogenesis and the effect of light dosage.
A. High Irradiance Reactions (HIR):
1. Plant photomorphogenic responses that require high light
levels and have action spectra different from that observed
with phytochrome. Most phytochrome responses show
saturation of red light at 200 J m-2. HIR needs about 100X
more red light energy in their responses.
2. HIR have three kinds of action spectra:
a. A single peak in the blue/UV-A spectral region
b. Two spectral peaks in the blue/UV-A and red regions
c. Three spectral peaks in the blue/UV-A, red, and far-red
regions.
3. When etiolated seedlings are exposed to light they turn
green (contain less type 1 phytochrome) and lose their
sensitivity to far-red light for HIR. HIR also do not show
red/far-red reversibility.
4. Etiolated angiosperm seedlings must activate cryptochrome in
order to become competent to respond to phytochrome. It
appears that cryptochrome activation is need before Pfr can
be expressed. Many cases have been recorded where both
cryptochrome and phytochrome cooperate in
photomorphogenesis.
B. Very Low Fluence Responses (VLFR): these plant responses
involve activation by very low levels of red light. But the
red light responses are not reversed by far-red light.
C. Low-fluence Responses (LFR): these are responses that are
typical of phytochrome. The red light response can be
reversed by far-red light. Also, the amount of light needed
is the same as those typically found with phytochrome action.
V. The effect of light in seed germination
A. Examples of light-dependent germination:
1. Seeds that respond to light are usually not from
domesticated stock. This would seem practical, single most
agricultural seed stocks have been developed so that they
are not dormant. The seed tends to be high is fat and small
in size. This would mean that a buried seed may not have
enough energy to germinate and push through the soil to the
surface. Therefor, the response to light ensures that the
seed is on the soil surface. Far-red light inhibits
germination while red light stimulates germination. Far-red
light would decrease the amount of Pfr phytochrome in the
seed tissues..
2. Photodormancy: seeds that need light to germinate.
Dormancy: seeds or buds that fail to grow when exposed to
adequate moisture, temperature, and air for growth.
B. Interactions between light and temperature in seed
photodormancy:
1. Temperature does not effect the interconversions between Pr
and Pfr. But, temperature does effect the processes that
are controlled by Pfr.
Example: Grand Rapids Lettuce (Lactuca sativa). Light
needed for seed germination. If the seed are treated with
35oC after light treatment they remain dormant. Cool
temperatures (10-15 oC) precludes a light treatment.
2. High temperatures decrease the Pfr level by increasing the
rate of reversion to Pr. Since Pfr is the active form of
phytochrome, reduction in its levels would inhibit a
positive response.
3. Seeds need to be partially or fully imbibed (water uptake)
before they will break dormancy. Phytochrome in the Pr form
is stable, so the seed needs only the proper moisture,
temperature, and air in order to respond to light and
germinate.
4. Seed germination that requires a light treatment will also
be controlled by the amount of Pfr the seed has acquired
from the maternal parent. Since chlorophyll absorbs red
light and prevents Pfr formation, seed embryos that ripen
covered by maternal tissues containing high amounts of
chlorophyll tend to require a light treatment for
germination. Seed embryo that ripen covered by maternal
tissues with little chlorophyll do not require a light
treatment.
5. The length of night and day during ripening affects
photodormancy. Long days (short nights) favor photodormant
seeds while short days (long nights) favor nondormant seeds.
C. Ecological aspects of photodormant seeds:
1. Photodormant Seed:
a. Assures that the seed is not covered by soil. The seed
will be able to photosynthesize soon after germination.
b. Assures that seed with germinate over several years as the
soil is disturbed. Prolongs longevity of the species.
2. Seed germination inhibited by light:
a. Seed germination is inhibited until the seed is buried in
the soil.
b. Assures that the seed is protected by soil and that
adequate moisture from the soil is available.
3. Phytochrome also provides the seed with clues as to the
amount of canopy cover the seed will face.
a. Leaves in a canopy transmit more far-red light than red
light. Far-red light usually inhibits seed germination in
light-requiring seed. The far-red light converts Pfr to
Pr. This would indicate to a seed that the canopy cover
is dense. If germination occurred, there may not be
enough light penetration for photosynthesis to occur in
the new seedling.
b. Plant species that require shade conditions for growth are
not affected by the amount of Pfr in their tissue (or the
amount of far-red light transmitted through a plant
canopy).
D. Red and far-red light are both absorbed by the hypocotyl-
radicle region of the seed. Phytochrome in the Pfr form
increases the growth potential of the radicle by decreasing
the water potential of the radicle cells so that they readily
absorb water from the soil and germinate. Germination is a
contest between the growth potential of the radicle and the
mechanical restrictiveness of the surrounding seed cell
layers. This mechanical restrictiveness can be either slight
or substantial. In response, the radicle may need little or
a great deal of force, caused by the growing tissues to break
dormancy. The amount of Pfr may determine how much the
radicle grows.
E. Hormones, Phytochrome, and Photodormancy:
1. Gibberellins: substitute for light or other environmental
requirements like temperature. Gibberellins induce the
weakening of the endosperm near the tip of the radicle.
This would allow radicle protrusion.
2. Cytokinins: can also substitute for a light treatment
3. Auxins: do not promote germination of photodormant or
nondormant seed. Can be inhibitory at high temperatures.
4. Ethylene: role is unclear; cannot break dormancy but does
effect other aspects of seed dormancy.
5. Abscisic Acid: retards germination
6. Pfr may break dormancy by causing synthesis of gibberellin
and cytokinins while inhibiting abscisic acid.
VI. Seedling establishment and vegetative growth of plants in
response to light.
A. Grass seedling (monocot) development in relation to light:
1. Light effects leaf growth and coleoptile elongation.
2. Phytochrome controls the unrolling of grass leaves. This
unrolling is possibly caused by gibberellin or cytokinin
whose levels are controlled by phytochrome (Pfr).
B. Dicot seedling development in relation to light:
1. Dicots form a hook near the stem apex after seed
germination. The seedling pushes through the soil pulling
the meristem after it.
2. Hook formation is an ethylene response. Red light promotes
hook opening. Hook opening occurs because ethylene
synthesis in the hook is inhibited.
3. Light also increases leaf-blade expansion, petiole
elongation, chlorophyll formation, and chloroplast
development.
C. Promotion of leaf growth by light in dicots is through HIR
(High Irradiance Reaction). The blue light causes cell
expansion by epidermal cell wall acidification. This loosens
the cell walls so that the leaf expands quickly under turgor
pressure. Pfr triggers chlorophyll formation and chloroplast
development. Pfr causes aminolevulinic acid (ALA), the
precursor of the pyrrole rings in chlorophyll, formation from
glutamic acid. Without blue and red light, the metabolic
pathway in formation of chlorophyll stops at
protochlorophyllide a (the immediate precursor of chlorophyll
a). Blue and red light are needed to add the phytol tail
(from isoprenoid pathway) to protochlorophyllide a to make
chlorophyll a. Chlorophyll a is then metabolized into
chlorophyll b.
VII. Affects of photomorphogenesis on vegetative growth in plants:
A. Stems do not grow as quickly during the day as they do at
night. Cryptochrome (blue light response) and phytochrome
(red response) are both required for stem elongation.
1. Plants growing under a leaf canopy receive more far-red
light than red light. This would cause the conversion of
Pfr to Pr. Thus the stems would elongate, carrying the
leaves (hopefully) toward the light.
2. Plants growing under a leaf canopy have reduced branching.
this allows more energy to be used in stem elongation to
carry the leaves to the top of the canopy.
3. Crop or greenhouse plants planted in outer rows are shorter
and more highly branched that those planted in inner rows.
This phenomenon is used in reforestation work were proper
spacing of trees can yield straight, knot free timber.
4. Shade tolerant plants usually do not respond to far-red
light stem elongation.
B. Far-red light reflection from one plant to another can also
effect plant growth. As competing plants grow, the reflected
far-red light can cause adjacent plants to elongate in a race
to keep ahead of their neighbors.
C. Tillering (branching at the base of grass stems) is promoted
by red light. Again, closely spaced plants that receive more
far-red light show less tillering. This allows the individual
plants to receive more light.
VIII. Photoperiods: plants respond to the time of day that light
is received. Phytochrome is the main plant pigment involved
in photoperiodic effects such as bud dormancy and production
of flowers and seeds. Long days promoted stem elongation and
short days lead to plant changes associated with autumn (bud
dormancy, frost-hardiness).
IX. Light effects on anthocyanin and flavonoid synthesis and
chloroplast arrangement.
A. Anthocyanin and flavonoid synthesis is promoted by light.
These substances are formed in specialized cells in the organ
in which they are produced (fruits, leaves, stems).
B. Both phytochrome and cryptochrome are the photoreceptors. UV-
B receptor may also be important in flavonoid synthesis.
C. Phototaxis: the movement of an organism or organelle in
response to light.
1. Under high light levels chloroplasts are lined up along the
radial walls of the cell. This allows the chloroplasts to
shade one another thereby reducing photooxidation. Under
low light the chloroplasts are distributed along the nearest
and farthest walls from the light source. This maximized
light absorption.
2. In angiosperms and mosses blue light, an hence cryptochrome,
is responsible for the phototaxic response of chloroplasts.
Phytochrome is not involved in this movement.
3. The chloroplast movement is affected by cytoplasmic
streaming which is controlled by the orientation of
microfilaments and microtubules of the cytoskeleton. Blue
light is absorbed by some component in the cells which
affects the cytoskeleton orientation.
X. Source of photomorphogenesis - how photoreceptors work:
A. There are two kinds of effects. The first is a fast membrane-
permeability effect, the second is a slower effect on gene
expression. Phytochrome and cryptochrome act on the
receptor-transduction system.
B. Pfr acts by changing membrane permeability. There is no
evidence that phytochrome is part of any plant membrane.
1. An example is the Tanada effect where root caps that are
treated with red-light develop a positive charge, causing
the root tips to stick to glass. H+ are transported from
the cytosol to cell walls by an ATPase in the plasma
membrane. Far-red light decreases this positive charge and
results in release of the root tips from the glass.
2. Nyctinastic movements of legumes leaves occurs through
changes in membrane permeability to potassium ions. The
electropotential across the plasma membrane is altered by
Pfr which allows leakage of potassium ions out of the
pulvini.
3. Ca2+ transport across the membranes is also affected by Pfr.
C. Phytochrome controls gene activation and deactivation. The
phytochrome effect depends on the cells involved and their
developmental state.
1. Stability of mRNA is one point of the gene control.
2. Pfr stimulates the Ca-calmodulin-dependent enzyme NAD+
kinase. Thus enzyme activation may be another way that
phytochrome controls gene expression.
3. Phytochrome may also act by a second-messenger in
transcription.
II. Phytochrome
A. A brief history:
1. Early work on the effect of flowering was done by W.W.
Garner and H.A. Allard in the 1920's. The found that the
duration of light and dark periods controlled flowering in
plants. One of the major research species was cocklebur
with required nights longer than a critical minimum to
flower (i.e. short day plants). It was found that different
wavelengths of light had different abilities to induce
flowering. Red light was the most effective in inducing
flowering.
2. Most of the research that first described phytochrome was
done between 1945 and 1960 at the U.S.D.A. in Beltsville,
Maryland by Harry Borthwick and Sterling Hendricks.
Borthwick and Hendricks worked with seed germination of Grand
Rapids lettuce seeds. They completed an action spectrum for
promotion of germination. There was a peak of seed
germination in the red wavelengths that could be reversed by
treatment with far-red light (wavelengths between 700-800
nm). They found that what ever light treatment was given
last determined whether the seeds germinated. This finding
laid the ground work for the discovery and isolation of
phytochrome.
B. Phytochrome and its properties:
1. There are two forms of phytochrome. The Pr form which
absorbs red light is blue in color. When Pr absorbs red
light it is converted to the Pfr form which is olive-green
in color. The Pfr form absorbs far-red light and is
subsequently converted into the Pr form. The Pr form is
considered the active form of phytochrome.
2. Absorption spectra for phytochrome:
a. Angiosperms: maxima in red wavelengths is 666 nm (Pr
absorbing form)
maxima in far-red wavelength is 730 nm (Pfr
absorbing form)
b. Found in angiosperms, gymnosperms, ferns, mosses,
liverworts, and green, red, and brown algae. The protein
moieties are different but the chromophore is the same in
all species. Phytochrome is synthesized in the Pr form.
Phytochrome is found in the cytosol and nucleus but does
not seem to be associated with any particular organelle.
3. Phytochrome is a chromoprotein. The protein portion is a
homodimer consisting of two identical polypeptides of 120
kDa. The chromophore is an open-chain tetrapyrrole
connected to the protein moiety through the sulfur of a
cysteine. When phytochrome absorbs red light there is a
cis-trans isomerization of the chromophore which causes
changes in the protein moiety. The changes in the protein
are responsible for the physiological changes.
4. When the Pr form absorbs red light about 85% of it is
converted to Pfr. Pfr is also converted back to Pr because
it can absorb a small amount of the red light.
5. There are two types of phytochrome (each can exists in the
Pr or Pfr form):
a. Type 1: found in etiolated seedlings
b. Type 2: found in green plants. Pr absorption maxima is
654 nm for red light and 724 nm for far-red light.
Several (at least five) different protein moieties have
been found.
c. Seedlings grown in the dark have much more type 1
phytochrome than type 2 phytochrome. This allows the
seedlings to respond to very low levels of red light.
Upon absorption of red light the majority of type 1
phytochrome is lost. There are three controlling factors
that govern this lose of type 1 phytochrome: (1) mRNA for
type 1 phytochrome is no longer produced, (2) mRNA for
type 1 phytochrome is highly unstable and is therefore
quickly degraded, (3) the type 1 phytochrome itself is
quickly degraded. Phytochrome self regulates the gene for
type 1 phytochrome production. This gene is active in the
dark, but is inactive in the light.
6. Evidence that support phytochrome as the pigment responsible
for photomorphogenesis are: (1) a correlation between the
absorption spectrum of phytochrome and the action spectra
for plant responses, (2) plant responses to red light can
be immediately reversed by exposure to far-red light, (3)
the irradiance level for the plant responses is quite low.
7. f (Psi)=Pfr/Pr + Pfr: the ratio of Pfr to the total amount
of phytochrome of both forms. This value is used to
describe the amount of phytochrome in a plant tissue. In
full sunlight the ratio of red to far-red photons is 1.1-
1.2. Pr absorbs red light more efficiently than Pfr
absorbs far-red light, which means that Pr will be converted
to Pfr quicker than Pfr can convert back to Pr. Therefor,
in sun light there is more Pfr than Pr and the f (Psi) value
is about 0.6.
8. If type 1 phytochrome found in etiolated plants receives a
brief red-light treatment, some of the Pfr formed will
gradually disappear over time. This disappearance of Pfr is
due to: (1) destruction or breakdown of Pfr by attachment to
ubiquitin (a 76 amino acid protein) which targets the Pfr
for degradation by proteases, or (2) dark reversion which
takes several hours. Dark reversion occurs in gymnosperms
and dicots, but not monocots.
9. Type 2 phytochrome does not undergo destruction or dark
reversion because it is more stable.
III. Cryptochrome:
In 1864 Julius vonSachs discovered that blue light caused
phototropism. Later it was found that near ultraviolet (UV)
light called UV-A radiation between 320 and 400 nm was also
involved in many plant developmental processes. The pigment
responsible for the absorption of light at these wavelengths is
called cryptochrome. Cryptochrome is a flavoprotein that is
associated with a cytochrome protein in the plasma membrane. Its
action spectrum maximum is found at 450 nm.
IV. Photomorphogenesis and the effect of light dosage.
A. High Irradiance Reactions (HIR):
1. Plant photomorphogenic responses that require high light
levels and have action spectra different from that observed
with phytochrome. Most phytochrome responses show
saturation of red light at 200 J m-2. HIR needs about 100X
more red light energy in their responses.
2. HIR have three kinds of action spectra:
a. A single peak in the blue/UV-A spectral region
b. Two spectral peaks in the blue/UV-A and red regions
c. Three spectral peaks in the blue/UV-A, red, and far-red
regions.
3. When etiolated seedlings are exposed to light they turn
green (contain less type 1 phytochrome) and lose their
sensitivity to far-red light for HIR. HIR also do not show
red/far-red reversibility.
4. Etiolated angiosperm seedlings must activate cryptochrome in
order to become competent to respond to phytochrome. It
appears that cryptochrome activation is need before Pfr can
be expressed. Many cases have been recorded where both
cryptochrome and phytochrome cooperate in
photomorphogenesis.
B. Very Low Fluence Responses (VLFR): these plant responses
involve activation by very low levels of red light. But the
red light responses are not reversed by far-red light.
C. Low-fluence Responses (LFR): these are responses that are
typical of phytochrome. The red light response can be
reversed by far-red light. Also, the amount of light needed
is the same as those typically found with phytochrome action.
V. The effect of light in seed germination
A. Examples of light-dependent germination:
1. Seeds that respond to light are usually not from
domesticated stock. This would seem practical, single most
agricultural seed stocks have been developed so that they
are not dormant. The seed tends to be high is fat and small
in size. This would mean that a buried seed may not have
enough energy to germinate and push through the soil to the
surface. Therefor, the response to light ensures that the
seed is on the soil surface. Far-red light inhibits
germination while red light stimulates germination. Far-red
light would decrease the amount of Pfr phytochrome in the
seed tissues..
2. Photodormancy: seeds that need light to germinate.
Dormancy: seeds or buds that fail to grow when exposed to
adequate moisture, temperature, and air for growth.
B. Interactions between light and temperature in seed
photodormancy:
1. Temperature does not effect the interconversions between Pr
and Pfr. But, temperature does effect the processes that
are controlled by Pfr.
Example: Grand Rapids Lettuce (Lactuca sativa). Light
needed for seed germination. If the seed are treated with
35oC after light treatment they remain dormant. Cool
temperatures (10-15 oC) precludes a light treatment.
2. High temperatures decrease the Pfr level by increasing the
rate of reversion to Pr. Since Pfr is the active form of
phytochrome, reduction in its levels would inhibit a
positive response.
3. Seeds need to be partially or fully imbibed (water uptake)
before they will break dormancy. Phytochrome in the Pr form
is stable, so the seed needs only the proper moisture,
temperature, and air in order to respond to light and
germinate.
4. Seed germination that requires a light treatment will also
be controlled by the amount of Pfr the seed has acquired
from the maternal parent. Since chlorophyll absorbs red
light and prevents Pfr formation, seed embryos that ripen
covered by maternal tissues containing high amounts of
chlorophyll tend to require a light treatment for
germination. Seed embryo that ripen covered by maternal
tissues with little chlorophyll do not require a light
treatment.
5. The length of night and day during ripening affects
photodormancy. Long days (short nights) favor photodormant
seeds while short days (long nights) favor nondormant seeds.
C. Ecological aspects of photodormant seeds:
1. Photodormant Seed:
a. Assures that the seed is not covered by soil. The seed
will be able to photosynthesize soon after germination.
b. Assures that seed with germinate over several years as the
soil is disturbed. Prolongs longevity of the species.
2. Seed germination inhibited by light:
a. Seed germination is inhibited until the seed is buried in
the soil.
b. Assures that the seed is protected by soil and that
adequate moisture from the soil is available.
3. Phytochrome also provides the seed with clues as to the
amount of canopy cover the seed will face.
a. Leaves in a canopy transmit more far-red light than red
light. Far-red light usually inhibits seed germination in
light-requiring seed. The far-red light converts Pfr to
Pr. This would indicate to a seed that the canopy cover
is dense. If germination occurred, there may not be
enough light penetration for photosynthesis to occur in
the new seedling.
b. Plant species that require shade conditions for growth are
not affected by the amount of Pfr in their tissue (or the
amount of far-red light transmitted through a plant
canopy).
D. Red and far-red light are both absorbed by the hypocotyl-
radicle region of the seed. Phytochrome in the Pfr form
increases the growth potential of the radicle by decreasing
the water potential of the radicle cells so that they readily
absorb water from the soil and germinate. Germination is a
contest between the growth potential of the radicle and the
mechanical restrictiveness of the surrounding seed cell
layers. This mechanical restrictiveness can be either slight
or substantial. In response, the radicle may need little or
a great deal of force, caused by the growing tissues to break
dormancy. The amount of Pfr may determine how much the
radicle grows.
E. Hormones, Phytochrome, and Photodormancy:
1. Gibberellins: substitute for light or other environmental
requirements like temperature. Gibberellins induce the
weakening of the endosperm near the tip of the radicle.
This would allow radicle protrusion.
2. Cytokinins: can also substitute for a light treatment
3. Auxins: do not promote germination of photodormant or
nondormant seed. Can be inhibitory at high temperatures.
4. Ethylene: role is unclear; cannot break dormancy but does
effect other aspects of seed dormancy.
5. Abscisic Acid: retards germination
6. Pfr may break dormancy by causing synthesis of gibberellin
and cytokinins while inhibiting abscisic acid.
VI. Seedling establishment and vegetative growth of plants in
response to light.
A. Grass seedling (monocot) development in relation to light:
1. Light effects leaf growth and coleoptile elongation.
2. Phytochrome controls the unrolling of grass leaves. This
unrolling is possibly caused by gibberellin or cytokinin
whose levels are controlled by phytochrome (Pfr).
B. Dicot seedling development in relation to light:
1. Dicots form a hook near the stem apex after seed
germination. The seedling pushes through the soil pulling
the meristem after it.
2. Hook formation is an ethylene response. Red light promotes
hook opening. Hook opening occurs because ethylene
synthesis in the hook is inhibited.
3. Light also increases leaf-blade expansion, petiole
elongation, chlorophyll formation, and chloroplast
development.
C. Promotion of leaf growth by light in dicots is through HIR
(High Irradiance Reaction). The blue light causes cell
expansion by epidermal cell wall acidification. This loosens
the cell walls so that the leaf expands quickly under turgor
pressure. Pfr triggers chlorophyll formation and chloroplast
development. Pfr causes aminolevulinic acid (ALA), the
precursor of the pyrrole rings in chlorophyll, formation from
glutamic acid. Without blue and red light, the metabolic
pathway in formation of chlorophyll stops at
protochlorophyllide a (the immediate precursor of chlorophyll
a). Blue and red light are needed to add the phytol tail
(from isoprenoid pathway) to protochlorophyllide a to make
chlorophyll a. Chlorophyll a is then metabolized into
chlorophyll b.
VII. Affects of photomorphogenesis on vegetative growth in plants:
A. Stems do not grow as quickly during the day as they do at
night. Cryptochrome (blue light response) and phytochrome
(red response) are both required for stem elongation.
1. Plants growing under a leaf canopy receive more far-red
light than red light. This would cause the conversion of
Pfr to Pr. Thus the stems would elongate, carrying the
leaves (hopefully) toward the light.
2. Plants growing under a leaf canopy have reduced branching.
this allows more energy to be used in stem elongation to
carry the leaves to the top of the canopy.
3. Crop or greenhouse plants planted in outer rows are shorter
and more highly branched that those planted in inner rows.
This phenomenon is used in reforestation work were proper
spacing of trees can yield straight, knot free timber.
4. Shade tolerant plants usually do not respond to far-red
light stem elongation.
B. Far-red light reflection from one plant to another can also
effect plant growth. As competing plants grow, the reflected
far-red light can cause adjacent plants to elongate in a race
to keep ahead of their neighbors.
C. Tillering (branching at the base of grass stems) is promoted
by red light. Again, closely spaced plants that receive more
far-red light show less tillering. This allows the individual
plants to receive more light.
VIII. Photoperiods: plants respond to the time of day that light
is received. Phytochrome is the main plant pigment involved
in photoperiodic effects such as bud dormancy and production
of flowers and seeds. Long days promoted stem elongation and
short days lead to plant changes associated with autumn (bud
dormancy, frost-hardiness).
IX. Light effects on anthocyanin and flavonoid synthesis and
chloroplast arrangement.
A. Anthocyanin and flavonoid synthesis is promoted by light.
These substances are formed in specialized cells in the organ
in which they are produced (fruits, leaves, stems).
B. Both phytochrome and cryptochrome are the photoreceptors. UV-
B receptor may also be important in flavonoid synthesis.
C. Phototaxis: the movement of an organism or organelle in
response to light.
1. Under high light levels chloroplasts are lined up along the
radial walls of the cell. This allows the chloroplasts to
shade one another thereby reducing photooxidation. Under
low light the chloroplasts are distributed along the nearest
and farthest walls from the light source. This maximized
light absorption.
2. In angiosperms and mosses blue light, an hence cryptochrome,
is responsible for the phototaxic response of chloroplasts.
Phytochrome is not involved in this movement.
3. The chloroplast movement is affected by cytoplasmic
streaming which is controlled by the orientation of
microfilaments and microtubules of the cytoskeleton. Blue
light is absorbed by some component in the cells which
affects the cytoskeleton orientation.
X. Source of photomorphogenesis - how photoreceptors work:
A. There are two kinds of effects. The first is a fast membrane-
permeability effect, the second is a slower effect on gene
expression. Phytochrome and cryptochrome act on the
receptor-transduction system.
B. Pfr acts by changing membrane permeability. There is no
evidence that phytochrome is part of any plant membrane.
1. An example is the Tanada effect where root caps that are
treated with red-light develop a positive charge, causing
the root tips to stick to glass. H+ are transported from
the cytosol to cell walls by an ATPase in the plasma
membrane. Far-red light decreases this positive charge and
results in release of the root tips from the glass.
2. Nyctinastic movements of legumes leaves occurs through
changes in membrane permeability to potassium ions. The
electropotential across the plasma membrane is altered by
Pfr which allows leakage of potassium ions out of the
pulvini.
3. Ca2+ transport across the membranes is also affected by Pfr.
C. Phytochrome controls gene activation and deactivation. The
phytochrome effect depends on the cells involved and their
developmental state.
1. Stability of mRNA is one point of the gene control.
2. Pfr stimulates the Ca-calmodulin-dependent enzyme NAD+
kinase. Thus enzyme activation may be another way that
phytochrome controls gene expression.
3. Phytochrome may also act by a second-messenger in
transcription.
 II. Phytochrome
A. A brief history:
1. Early work on the effect of flowering was done by W.W.
Garner and H.A. Allard in the 1920's. The found that the
duration of light and dark periods controlled flowering in
plants. One of the major research species was cocklebur
with required nights longer than a critical minimum to
flower (i.e. short day plants). It was found that different
wavelengths of light had different abilities to induce
flowering. Red light was the most effective in inducing
flowering.
2. Most of the research that first described phytochrome was
done between 1945 and 1960 at the U.S.D.A. in Beltsville,
Maryland by Harry Borthwick and Sterling Hendricks.
Borthwick and Hendricks worked with seed germination of Grand
Rapids lettuce seeds. They completed an action spectrum for
promotion of germination. There was a peak of seed
germination in the red wavelengths that could be reversed by
treatment with far-red light (wavelengths between 700-800
nm). They found that what ever light treatment was given
last determined whether the seeds germinated. This finding
laid the ground work for the discovery and isolation of
phytochrome.
B. Phytochrome and its properties:
1. There are two forms of phytochrome. The Pr form which
absorbs red light is blue in color. When Pr absorbs red
light it is converted to the Pfr form which is olive-green
in color. The Pfr form absorbs far-red light and is
subsequently converted into the Pr form. The Pr form is
considered the active form of phytochrome.
2. Absorption spectra for phytochrome:
a. Angiosperms: maxima in red wavelengths is 666 nm (Pr
absorbing form)
maxima in far-red wavelength is 730 nm (Pfr
absorbing form)
b. Found in angiosperms, gymnosperms, ferns, mosses,
liverworts, and green, red, and brown algae. The protein
moieties are different but the chromophore is the same in
all species. Phytochrome is synthesized in the Pr form.
Phytochrome is found in the cytosol and nucleus but does
not seem to be associated with any particular organelle.
3. Phytochrome is a chromoprotein. The protein portion is a
homodimer consisting of two identical polypeptides of 120
kDa. The chromophore is an open-chain tetrapyrrole
connected to the protein moiety through the sulfur of a
cysteine. When phytochrome absorbs red light there is a
cis-trans isomerization of the chromophore which causes
changes in the protein moiety. The changes in the protein
are responsible for the physiological changes.
4. When the Pr form absorbs red light about 85% of it is
converted to Pfr. Pfr is also converted back to Pr because
it can absorb a small amount of the red light.
5. There are two types of phytochrome (each can exists in the
Pr or Pfr form):
a. Type 1: found in etiolated seedlings
b. Type 2: found in green plants. Pr absorption maxima is
654 nm for red light and 724 nm for far-red light.
Several (at least five) different protein moieties have
been found.
c. Seedlings grown in the dark have much more type 1
phytochrome than type 2 phytochrome. This allows the
seedlings to respond to very low levels of red light.
Upon absorption of red light the majority of type 1
phytochrome is lost. There are three controlling factors
that govern this lose of type 1 phytochrome: (1) mRNA for
type 1 phytochrome is no longer produced, (2) mRNA for
type 1 phytochrome is highly unstable and is therefore
quickly degraded, (3) the type 1 phytochrome itself is
quickly degraded. Phytochrome self regulates the gene for
type 1 phytochrome production. This gene is active in the
dark, but is inactive in the light.
6. Evidence that support phytochrome as the pigment responsible
for photomorphogenesis are: (1) a correlation between the
absorption spectrum of phytochrome and the action spectra
for plant responses, (2) plant responses to red light can
be immediately reversed by exposure to far-red light, (3)
the irradiance level for the plant responses is quite low.
7. f (Psi)=Pfr/Pr + Pfr: the ratio of Pfr to the total amount
of phytochrome of both forms. This value is used to
describe the amount of phytochrome in a plant tissue. In
full sunlight the ratio of red to far-red photons is 1.1-
1.2. Pr absorbs red light more efficiently than Pfr
absorbs far-red light, which means that Pr will be converted
to Pfr quicker than Pfr can convert back to Pr. Therefor,
in sun light there is more Pfr than Pr and the f (Psi) value
is about 0.6.
8. If type 1 phytochrome found in etiolated plants receives a
brief red-light treatment, some of the Pfr formed will
gradually disappear over time. This disappearance of Pfr is
due to: (1) destruction or breakdown of Pfr by attachment to
ubiquitin (a 76 amino acid protein) which targets the Pfr
for degradation by proteases, or (2) dark reversion which
takes several hours. Dark reversion occurs in gymnosperms
and dicots, but not monocots.
9. Type 2 phytochrome does not undergo destruction or dark
reversion because it is more stable.
III. Cryptochrome:
In 1864 Julius vonSachs discovered that blue light caused
phototropism. Later it was found that near ultraviolet (UV)
light called UV-A radiation between 320 and 400 nm was also
involved in many plant developmental processes. The pigment
responsible for the absorption of light at these wavelengths is
called cryptochrome. Cryptochrome is a flavoprotein that is
associated with a cytochrome protein in the plasma membrane. Its
action spectrum maximum is found at 450 nm.
IV. Photomorphogenesis and the effect of light dosage.
A. High Irradiance Reactions (HIR):
1. Plant photomorphogenic responses that require high light
levels and have action spectra different from that observed
with phytochrome. Most phytochrome responses show
saturation of red light at 200 J m-2. HIR needs about 100X
more red light energy in their responses.
2. HIR have three kinds of action spectra:
a. A single peak in the blue/UV-A spectral region
b. Two spectral peaks in the blue/UV-A and red regions
c. Three spectral peaks in the blue/UV-A, red, and far-red
regions.
3. When etiolated seedlings are exposed to light they turn
green (contain less type 1 phytochrome) and lose their
sensitivity to far-red light for HIR. HIR also do not show
red/far-red reversibility.
4. Etiolated angiosperm seedlings must activate cryptochrome in
order to become competent to respond to phytochrome. It
appears that cryptochrome activation is need before Pfr can
be expressed. Many cases have been recorded where both
cryptochrome and phytochrome cooperate in
photomorphogenesis.
B. Very Low Fluence Responses (VLFR): these plant responses
involve activation by very low levels of red light. But the
red light responses are not reversed by far-red light.
C. Low-fluence Responses (LFR): these are responses that are
typical of phytochrome. The red light response can be
reversed by far-red light. Also, the amount of light needed
is the same as those typically found with phytochrome action.
V. The effect of light in seed germination
A. Examples of light-dependent germination:
1. Seeds that respond to light are usually not from
domesticated stock. This would seem practical, single most
agricultural seed stocks have been developed so that they
are not dormant. The seed tends to be high is fat and small
in size. This would mean that a buried seed may not have
enough energy to germinate and push through the soil to the
surface. Therefor, the response to light ensures that the
seed is on the soil surface. Far-red light inhibits
germination while red light stimulates germination. Far-red
light would decrease the amount of Pfr phytochrome in the
seed tissues..
2. Photodormancy: seeds that need light to germinate.
Dormancy: seeds or buds that fail to grow when exposed to
adequate moisture, temperature, and air for growth.
B. Interactions between light and temperature in seed
photodormancy:
1. Temperature does not effect the interconversions between Pr
and Pfr. But, temperature does effect the processes that
are controlled by Pfr.
Example: Grand Rapids Lettuce (Lactuca sativa). Light
needed for seed germination. If the seed are treated with
35oC after light treatment they remain dormant. Cool
temperatures (10-15 oC) precludes a light treatment.
2. High temperatures decrease the Pfr level by increasing the
rate of reversion to Pr. Since Pfr is the active form of
phytochrome, reduction in its levels would inhibit a
positive response.
3. Seeds need to be partially or fully imbibed (water uptake)
before they will break dormancy. Phytochrome in the Pr form
is stable, so the seed needs only the proper moisture,
temperature, and air in order to respond to light and
germinate.
4. Seed germination that requires a light treatment will also
be controlled by the amount of Pfr the seed has acquired
from the maternal parent. Since chlorophyll absorbs red
light and prevents Pfr formation, seed embryos that ripen
covered by maternal tissues containing high amounts of
chlorophyll tend to require a light treatment for
germination. Seed embryo that ripen covered by maternal
tissues with little chlorophyll do not require a light
treatment.
5. The length of night and day during ripening affects
photodormancy. Long days (short nights) favor photodormant
seeds while short days (long nights) favor nondormant seeds.
C. Ecological aspects of photodormant seeds:
1. Photodormant Seed:
a. Assures that the seed is not covered by soil. The seed
will be able to photosynthesize soon after germination.
b. Assures that seed with germinate over several years as the
soil is disturbed. Prolongs longevity of the species.
2. Seed germination inhibited by light:
a. Seed germination is inhibited until the seed is buried in
the soil.
b. Assures that the seed is protected by soil and that
adequate moisture from the soil is available.
3. Phytochrome also provides the seed with clues as to the
amount of canopy cover the seed will face.
a. Leaves in a canopy transmit more far-red light than red
light. Far-red light usually inhibits seed germination in
light-requiring seed. The far-red light converts Pfr to
Pr. This would indicate to a seed that the canopy cover
is dense. If germination occurred, there may not be
enough light penetration for photosynthesis to occur in
the new seedling.
b. Plant species that require shade conditions for growth are
not affected by the amount of Pfr in their tissue (or the
amount of far-red light transmitted through a plant
canopy).
D. Red and far-red light are both absorbed by the hypocotyl-
radicle region of the seed. Phytochrome in the Pfr form
increases the growth potential of the radicle by decreasing
the water potential of the radicle cells so that they readily
absorb water from the soil and germinate. Germination is a
contest between the growth potential of the radicle and the
mechanical restrictiveness of the surrounding seed cell
layers. This mechanical restrictiveness can be either slight
or substantial. In response, the radicle may need little or
a great deal of force, caused by the growing tissues to break
dormancy. The amount of Pfr may determine how much the
radicle grows.
E. Hormones, Phytochrome, and Photodormancy:
1. Gibberellins: substitute for light or other environmental
requirements like temperature. Gibberellins induce the
weakening of the endosperm near the tip of the radicle.
This would allow radicle protrusion.
2. Cytokinins: can also substitute for a light treatment
3. Auxins: do not promote germination of photodormant or
nondormant seed. Can be inhibitory at high temperatures.
4. Ethylene: role is unclear; cannot break dormancy but does
effect other aspects of seed dormancy.
5. Abscisic Acid: retards germination
6. Pfr may break dormancy by causing synthesis of gibberellin
and cytokinins while inhibiting abscisic acid.
VI. Seedling establishment and vegetative growth of plants in
response to light.
A. Grass seedling (monocot) development in relation to light:
1. Light effects leaf growth and coleoptile elongation.
2. Phytochrome controls the unrolling of grass leaves. This
unrolling is possibly caused by gibberellin or cytokinin
whose levels are controlled by phytochrome (Pfr).
B. Dicot seedling development in relation to light:
1. Dicots form a hook near the stem apex after seed
germination. The seedling pushes through the soil pulling
the meristem after it.
2. Hook formation is an ethylene response. Red light promotes
hook opening. Hook opening occurs because ethylene
synthesis in the hook is inhibited.
3. Light also increases leaf-blade expansion, petiole
elongation, chlorophyll formation, and chloroplast
development.
C. Promotion of leaf growth by light in dicots is through HIR
(High Irradiance Reaction). The blue light causes cell
expansion by epidermal cell wall acidification. This loosens
the cell walls so that the leaf expands quickly under turgor
pressure. Pfr triggers chlorophyll formation and chloroplast
development. Pfr causes aminolevulinic acid (ALA), the
precursor of the pyrrole rings in chlorophyll, formation from
glutamic acid. Without blue and red light, the metabolic
pathway in formation of chlorophyll stops at
protochlorophyllide a (the immediate precursor of chlorophyll
a). Blue and red light are needed to add the phytol tail
(from isoprenoid pathway) to protochlorophyllide a to make
chlorophyll a. Chlorophyll a is then metabolized into
chlorophyll b.
VII. Affects of photomorphogenesis on vegetative growth in plants:
A. Stems do not grow as quickly during the day as they do at
night. Cryptochrome (blue light response) and phytochrome
(red response) are both required for stem elongation.
1. Plants growing under a leaf canopy receive more far-red
light than red light. This would cause the conversion of
Pfr to Pr. Thus the stems would elongate, carrying the
leaves (hopefully) toward the light.
2. Plants growing under a leaf canopy have reduced branching.
this allows more energy to be used in stem elongation to
carry the leaves to the top of the canopy.
3. Crop or greenhouse plants planted in outer rows are shorter
and more highly branched that those planted in inner rows.
This phenomenon is used in reforestation work were proper
spacing of trees can yield straight, knot free timber.
4. Shade tolerant plants usually do not respond to far-red
light stem elongation.
B. Far-red light reflection from one plant to another can also
effect plant growth. As competing plants grow, the reflected
far-red light can cause adjacent plants to elongate in a race
to keep ahead of their neighbors.
C. Tillering (branching at the base of grass stems) is promoted
by red light. Again, closely spaced plants that receive more
far-red light show less tillering. This allows the individual
plants to receive more light.
VIII. Photoperiods: plants respond to the time of day that light
is received. Phytochrome is the main plant pigment involved
in photoperiodic effects such as bud dormancy and production
of flowers and seeds. Long days promoted stem elongation and
short days lead to plant changes associated with autumn (bud
dormancy, frost-hardiness).
IX. Light effects on anthocyanin and flavonoid synthesis and
chloroplast arrangement.
A. Anthocyanin and flavonoid synthesis is promoted by light.
These substances are formed in specialized cells in the organ
in which they are produced (fruits, leaves, stems).
B. Both phytochrome and cryptochrome are the photoreceptors. UV-
B receptor may also be important in flavonoid synthesis.
C. Phototaxis: the movement of an organism or organelle in
response to light.
1. Under high light levels chloroplasts are lined up along the
radial walls of the cell. This allows the chloroplasts to
shade one another thereby reducing photooxidation. Under
low light the chloroplasts are distributed along the nearest
and farthest walls from the light source. This maximized
light absorption.
2. In angiosperms and mosses blue light, an hence cryptochrome,
is responsible for the phototaxic response of chloroplasts.
Phytochrome is not involved in this movement.
3. The chloroplast movement is affected by cytoplasmic
streaming which is controlled by the orientation of
microfilaments and microtubules of the cytoskeleton. Blue
light is absorbed by some component in the cells which
affects the cytoskeleton orientation.
X. Source of photomorphogenesis - how photoreceptors work:
A. There are two kinds of effects. The first is a fast membrane-
permeability effect, the second is a slower effect on gene
expression. Phytochrome and cryptochrome act on the
receptor-transduction system.
B. Pfr acts by changing membrane permeability. There is no
evidence that phytochrome is part of any plant membrane.
1. An example is the Tanada effect where root caps that are
treated with red-light develop a positive charge, causing
the root tips to stick to glass. H+ are transported from
the cytosol to cell walls by an ATPase in the plasma
membrane. Far-red light decreases this positive charge and
results in release of the root tips from the glass.
2. Nyctinastic movements of legumes leaves occurs through
changes in membrane permeability to potassium ions. The
electropotential across the plasma membrane is altered by
Pfr which allows leakage of potassium ions out of the
pulvini.
3. Ca2+ transport across the membranes is also affected by Pfr.
C. Phytochrome controls gene activation and deactivation. The
phytochrome effect depends on the cells involved and their
developmental state.
1. Stability of mRNA is one point of the gene control.
2. Pfr stimulates the Ca-calmodulin-dependent enzyme NAD+
kinase. Thus enzyme activation may be another way that
phytochrome controls gene expression.
3. Phytochrome may also act by a second-messenger in
transcription.
II. Phytochrome
A. A brief history:
1. Early work on the effect of flowering was done by W.W.
Garner and H.A. Allard in the 1920's. The found that the
duration of light and dark periods controlled flowering in
plants. One of the major research species was cocklebur
with required nights longer than a critical minimum to
flower (i.e. short day plants). It was found that different
wavelengths of light had different abilities to induce
flowering. Red light was the most effective in inducing
flowering.
2. Most of the research that first described phytochrome was
done between 1945 and 1960 at the U.S.D.A. in Beltsville,
Maryland by Harry Borthwick and Sterling Hendricks.
Borthwick and Hendricks worked with seed germination of Grand
Rapids lettuce seeds. They completed an action spectrum for
promotion of germination. There was a peak of seed
germination in the red wavelengths that could be reversed by
treatment with far-red light (wavelengths between 700-800
nm). They found that what ever light treatment was given
last determined whether the seeds germinated. This finding
laid the ground work for the discovery and isolation of
phytochrome.
B. Phytochrome and its properties:
1. There are two forms of phytochrome. The Pr form which
absorbs red light is blue in color. When Pr absorbs red
light it is converted to the Pfr form which is olive-green
in color. The Pfr form absorbs far-red light and is
subsequently converted into the Pr form. The Pr form is
considered the active form of phytochrome.
2. Absorption spectra for phytochrome:
a. Angiosperms: maxima in red wavelengths is 666 nm (Pr
absorbing form)
maxima in far-red wavelength is 730 nm (Pfr
absorbing form)
b. Found in angiosperms, gymnosperms, ferns, mosses,
liverworts, and green, red, and brown algae. The protein
moieties are different but the chromophore is the same in
all species. Phytochrome is synthesized in the Pr form.
Phytochrome is found in the cytosol and nucleus but does
not seem to be associated with any particular organelle.
3. Phytochrome is a chromoprotein. The protein portion is a
homodimer consisting of two identical polypeptides of 120
kDa. The chromophore is an open-chain tetrapyrrole
connected to the protein moiety through the sulfur of a
cysteine. When phytochrome absorbs red light there is a
cis-trans isomerization of the chromophore which causes
changes in the protein moiety. The changes in the protein
are responsible for the physiological changes.
4. When the Pr form absorbs red light about 85% of it is
converted to Pfr. Pfr is also converted back to Pr because
it can absorb a small amount of the red light.
5. There are two types of phytochrome (each can exists in the
Pr or Pfr form):
a. Type 1: found in etiolated seedlings
b. Type 2: found in green plants. Pr absorption maxima is
654 nm for red light and 724 nm for far-red light.
Several (at least five) different protein moieties have
been found.
c. Seedlings grown in the dark have much more type 1
phytochrome than type 2 phytochrome. This allows the
seedlings to respond to very low levels of red light.
Upon absorption of red light the majority of type 1
phytochrome is lost. There are three controlling factors
that govern this lose of type 1 phytochrome: (1) mRNA for
type 1 phytochrome is no longer produced, (2) mRNA for
type 1 phytochrome is highly unstable and is therefore
quickly degraded, (3) the type 1 phytochrome itself is
quickly degraded. Phytochrome self regulates the gene for
type 1 phytochrome production. This gene is active in the
dark, but is inactive in the light.
6. Evidence that support phytochrome as the pigment responsible
for photomorphogenesis are: (1) a correlation between the
absorption spectrum of phytochrome and the action spectra
for plant responses, (2) plant responses to red light can
be immediately reversed by exposure to far-red light, (3)
the irradiance level for the plant responses is quite low.
7. f (Psi)=Pfr/Pr + Pfr: the ratio of Pfr to the total amount
of phytochrome of both forms. This value is used to
describe the amount of phytochrome in a plant tissue. In
full sunlight the ratio of red to far-red photons is 1.1-
1.2. Pr absorbs red light more efficiently than Pfr
absorbs far-red light, which means that Pr will be converted
to Pfr quicker than Pfr can convert back to Pr. Therefor,
in sun light there is more Pfr than Pr and the f (Psi) value
is about 0.6.
8. If type 1 phytochrome found in etiolated plants receives a
brief red-light treatment, some of the Pfr formed will
gradually disappear over time. This disappearance of Pfr is
due to: (1) destruction or breakdown of Pfr by attachment to
ubiquitin (a 76 amino acid protein) which targets the Pfr
for degradation by proteases, or (2) dark reversion which
takes several hours. Dark reversion occurs in gymnosperms
and dicots, but not monocots.
9. Type 2 phytochrome does not undergo destruction or dark
reversion because it is more stable.
III. Cryptochrome:
In 1864 Julius vonSachs discovered that blue light caused
phototropism. Later it was found that near ultraviolet (UV)
light called UV-A radiation between 320 and 400 nm was also
involved in many plant developmental processes. The pigment
responsible for the absorption of light at these wavelengths is
called cryptochrome. Cryptochrome is a flavoprotein that is
associated with a cytochrome protein in the plasma membrane. Its
action spectrum maximum is found at 450 nm.
IV. Photomorphogenesis and the effect of light dosage.
A. High Irradiance Reactions (HIR):
1. Plant photomorphogenic responses that require high light
levels and have action spectra different from that observed
with phytochrome. Most phytochrome responses show
saturation of red light at 200 J m-2. HIR needs about 100X
more red light energy in their responses.
2. HIR have three kinds of action spectra:
a. A single peak in the blue/UV-A spectral region
b. Two spectral peaks in the blue/UV-A and red regions
c. Three spectral peaks in the blue/UV-A, red, and far-red
regions.
3. When etiolated seedlings are exposed to light they turn
green (contain less type 1 phytochrome) and lose their
sensitivity to far-red light for HIR. HIR also do not show
red/far-red reversibility.
4. Etiolated angiosperm seedlings must activate cryptochrome in
order to become competent to respond to phytochrome. It
appears that cryptochrome activation is need before Pfr can
be expressed. Many cases have been recorded where both
cryptochrome and phytochrome cooperate in
photomorphogenesis.
B. Very Low Fluence Responses (VLFR): these plant responses
involve activation by very low levels of red light. But the
red light responses are not reversed by far-red light.
C. Low-fluence Responses (LFR): these are responses that are
typical of phytochrome. The red light response can be
reversed by far-red light. Also, the amount of light needed
is the same as those typically found with phytochrome action.
V. The effect of light in seed germination
A. Examples of light-dependent germination:
1. Seeds that respond to light are usually not from
domesticated stock. This would seem practical, single most
agricultural seed stocks have been developed so that they
are not dormant. The seed tends to be high is fat and small
in size. This would mean that a buried seed may not have
enough energy to germinate and push through the soil to the
surface. Therefor, the response to light ensures that the
seed is on the soil surface. Far-red light inhibits
germination while red light stimulates germination. Far-red
light would decrease the amount of Pfr phytochrome in the
seed tissues..
2. Photodormancy: seeds that need light to germinate.
Dormancy: seeds or buds that fail to grow when exposed to
adequate moisture, temperature, and air for growth.
B. Interactions between light and temperature in seed
photodormancy:
1. Temperature does not effect the interconversions between Pr
and Pfr. But, temperature does effect the processes that
are controlled by Pfr.
Example: Grand Rapids Lettuce (Lactuca sativa). Light
needed for seed germination. If the seed are treated with
35oC after light treatment they remain dormant. Cool
temperatures (10-15 oC) precludes a light treatment.
2. High temperatures decrease the Pfr level by increasing the
rate of reversion to Pr. Since Pfr is the active form of
phytochrome, reduction in its levels would inhibit a
positive response.
3. Seeds need to be partially or fully imbibed (water uptake)
before they will break dormancy. Phytochrome in the Pr form
is stable, so the seed needs only the proper moisture,
temperature, and air in order to respond to light and
germinate.
4. Seed germination that requires a light treatment will also
be controlled by the amount of Pfr the seed has acquired
from the maternal parent. Since chlorophyll absorbs red
light and prevents Pfr formation, seed embryos that ripen
covered by maternal tissues containing high amounts of
chlorophyll tend to require a light treatment for
germination. Seed embryo that ripen covered by maternal
tissues with little chlorophyll do not require a light
treatment.
5. The length of night and day during ripening affects
photodormancy. Long days (short nights) favor photodormant
seeds while short days (long nights) favor nondormant seeds.
C. Ecological aspects of photodormant seeds:
1. Photodormant Seed:
a. Assures that the seed is not covered by soil. The seed
will be able to photosynthesize soon after germination.
b. Assures that seed with germinate over several years as the
soil is disturbed. Prolongs longevity of the species.
2. Seed germination inhibited by light:
a. Seed germination is inhibited until the seed is buried in
the soil.
b. Assures that the seed is protected by soil and that
adequate moisture from the soil is available.
3. Phytochrome also provides the seed with clues as to the
amount of canopy cover the seed will face.
a. Leaves in a canopy transmit more far-red light than red
light. Far-red light usually inhibits seed germination in
light-requiring seed. The far-red light converts Pfr to
Pr. This would indicate to a seed that the canopy cover
is dense. If germination occurred, there may not be
enough light penetration for photosynthesis to occur in
the new seedling.
b. Plant species that require shade conditions for growth are
not affected by the amount of Pfr in their tissue (or the
amount of far-red light transmitted through a plant
canopy).
D. Red and far-red light are both absorbed by the hypocotyl-
radicle region of the seed. Phytochrome in the Pfr form
increases the growth potential of the radicle by decreasing
the water potential of the radicle cells so that they readily
absorb water from the soil and germinate. Germination is a
contest between the growth potential of the radicle and the
mechanical restrictiveness of the surrounding seed cell
layers. This mechanical restrictiveness can be either slight
or substantial. In response, the radicle may need little or
a great deal of force, caused by the growing tissues to break
dormancy. The amount of Pfr may determine how much the
radicle grows.
E. Hormones, Phytochrome, and Photodormancy:
1. Gibberellins: substitute for light or other environmental
requirements like temperature. Gibberellins induce the
weakening of the endosperm near the tip of the radicle.
This would allow radicle protrusion.
2. Cytokinins: can also substitute for a light treatment
3. Auxins: do not promote germination of photodormant or
nondormant seed. Can be inhibitory at high temperatures.
4. Ethylene: role is unclear; cannot break dormancy but does
effect other aspects of seed dormancy.
5. Abscisic Acid: retards germination
6. Pfr may break dormancy by causing synthesis of gibberellin
and cytokinins while inhibiting abscisic acid.
VI. Seedling establishment and vegetative growth of plants in
response to light.
A. Grass seedling (monocot) development in relation to light:
1. Light effects leaf growth and coleoptile elongation.
2. Phytochrome controls the unrolling of grass leaves. This
unrolling is possibly caused by gibberellin or cytokinin
whose levels are controlled by phytochrome (Pfr).
B. Dicot seedling development in relation to light:
1. Dicots form a hook near the stem apex after seed
germination. The seedling pushes through the soil pulling
the meristem after it.
2. Hook formation is an ethylene response. Red light promotes
hook opening. Hook opening occurs because ethylene
synthesis in the hook is inhibited.
3. Light also increases leaf-blade expansion, petiole
elongation, chlorophyll formation, and chloroplast
development.
C. Promotion of leaf growth by light in dicots is through HIR
(High Irradiance Reaction). The blue light causes cell
expansion by epidermal cell wall acidification. This loosens
the cell walls so that the leaf expands quickly under turgor
pressure. Pfr triggers chlorophyll formation and chloroplast
development. Pfr causes aminolevulinic acid (ALA), the
precursor of the pyrrole rings in chlorophyll, formation from
glutamic acid. Without blue and red light, the metabolic
pathway in formation of chlorophyll stops at
protochlorophyllide a (the immediate precursor of chlorophyll
a). Blue and red light are needed to add the phytol tail
(from isoprenoid pathway) to protochlorophyllide a to make
chlorophyll a. Chlorophyll a is then metabolized into
chlorophyll b.
VII. Affects of photomorphogenesis on vegetative growth in plants:
A. Stems do not grow as quickly during the day as they do at
night. Cryptochrome (blue light response) and phytochrome
(red response) are both required for stem elongation.
1. Plants growing under a leaf canopy receive more far-red
light than red light. This would cause the conversion of
Pfr to Pr. Thus the stems would elongate, carrying the
leaves (hopefully) toward the light.
2. Plants growing under a leaf canopy have reduced branching.
this allows more energy to be used in stem elongation to
carry the leaves to the top of the canopy.
3. Crop or greenhouse plants planted in outer rows are shorter
and more highly branched that those planted in inner rows.
This phenomenon is used in reforestation work were proper
spacing of trees can yield straight, knot free timber.
4. Shade tolerant plants usually do not respond to far-red
light stem elongation.
B. Far-red light reflection from one plant to another can also
effect plant growth. As competing plants grow, the reflected
far-red light can cause adjacent plants to elongate in a race
to keep ahead of their neighbors.
C. Tillering (branching at the base of grass stems) is promoted
by red light. Again, closely spaced plants that receive more
far-red light show less tillering. This allows the individual
plants to receive more light.
VIII. Photoperiods: plants respond to the time of day that light
is received. Phytochrome is the main plant pigment involved
in photoperiodic effects such as bud dormancy and production
of flowers and seeds. Long days promoted stem elongation and
short days lead to plant changes associated with autumn (bud
dormancy, frost-hardiness).
IX. Light effects on anthocyanin and flavonoid synthesis and
chloroplast arrangement.
A. Anthocyanin and flavonoid synthesis is promoted by light.
These substances are formed in specialized cells in the organ
in which they are produced (fruits, leaves, stems).
B. Both phytochrome and cryptochrome are the photoreceptors. UV-
B receptor may also be important in flavonoid synthesis.
C. Phototaxis: the movement of an organism or organelle in
response to light.
1. Under high light levels chloroplasts are lined up along the
radial walls of the cell. This allows the chloroplasts to
shade one another thereby reducing photooxidation. Under
low light the chloroplasts are distributed along the nearest
and farthest walls from the light source. This maximized
light absorption.
2. In angiosperms and mosses blue light, an hence cryptochrome,
is responsible for the phototaxic response of chloroplasts.
Phytochrome is not involved in this movement.
3. The chloroplast movement is affected by cytoplasmic
streaming which is controlled by the orientation of
microfilaments and microtubules of the cytoskeleton. Blue
light is absorbed by some component in the cells which
affects the cytoskeleton orientation.
X. Source of photomorphogenesis - how photoreceptors work:
A. There are two kinds of effects. The first is a fast membrane-
permeability effect, the second is a slower effect on gene
expression. Phytochrome and cryptochrome act on the
receptor-transduction system.
B. Pfr acts by changing membrane permeability. There is no
evidence that phytochrome is part of any plant membrane.
1. An example is the Tanada effect where root caps that are
treated with red-light develop a positive charge, causing
the root tips to stick to glass. H+ are transported from
the cytosol to cell walls by an ATPase in the plasma
membrane. Far-red light decreases this positive charge and
results in release of the root tips from the glass.
2. Nyctinastic movements of legumes leaves occurs through
changes in membrane permeability to potassium ions. The
electropotential across the plasma membrane is altered by
Pfr which allows leakage of potassium ions out of the
pulvini.
3. Ca2+ transport across the membranes is also affected by Pfr.
C. Phytochrome controls gene activation and deactivation. The
phytochrome effect depends on the cells involved and their
developmental state.
1. Stability of mRNA is one point of the gene control.
2. Pfr stimulates the Ca-calmodulin-dependent enzyme NAD+
kinase. Thus enzyme activation may be another way that
phytochrome controls gene expression.
3. Phytochrome may also act by a second-messenger in
transcription.