Cell Biology
I. Overview
II. Membranes: How
Matter Get in and Out of Cells
III. Cellular Respiration
IV. Photosynthesis
V. DNA and Chromosomes Structure
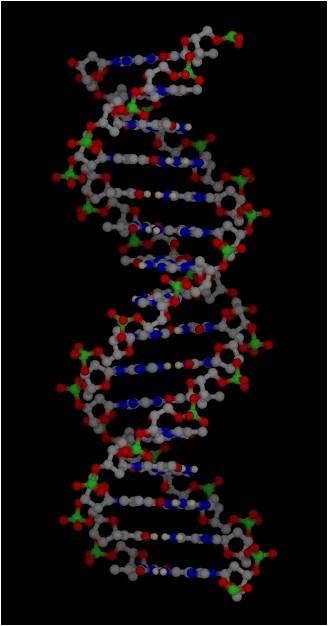 Much of the energy harvested by a cell is used to make proteins. However, in order to understand protein synthesis, we must first describe the structure of DNA. DNA is the 'recipe' for proteins, so we need to take a little detour on our jounrey of how a cell works to describe the structure of DNA and chromosomes. DNA
is the genetic material in all forms of life (eubacteria, archaea, protists,
plants, fungi, and animals). Those quasi-living viruses vary in their genetic
material. Some have double-stranded DNA (ds-DNA) like living systems, while
others have ss-DNA, ss-RNA, and ds-RNA. RNA performs a wide array of functions
in living systems. Many of these functions have only been discovered in the
last few years.
Much of the energy harvested by a cell is used to make proteins. However, in order to understand protein synthesis, we must first describe the structure of DNA. DNA is the 'recipe' for proteins, so we need to take a little detour on our jounrey of how a cell works to describe the structure of DNA and chromosomes. DNA
is the genetic material in all forms of life (eubacteria, archaea, protists,
plants, fungi, and animals). Those quasi-living viruses vary in their genetic
material. Some have double-stranded DNA (ds-DNA) like living systems, while
others have ss-DNA, ss-RNA, and ds-RNA. RNA performs a wide array of functions
in living systems. Many of these functions have only been discovered in the
last few years.
With the rediscovery of Mendel's
laws in 1900, and the proposal of the "Chromosomal Theory of Inheritance"
by Sutton and Boveri in 1902, there was a natural curiosity in determining the
structure of chromosomes and the way they functioned to influence heritable
characteristics. It is a fascinating story and it continues to this day; we
are still finding new ways that the genetic system regulates the activities
of the cell. Although this story will be covered in appropriate detail in BIO
221: Genetics, here is a quick overview.
Chemical digestions of nuclei Meischner
(1868) revealed an acidic fraction, which he termed nuclein. Chargaff's digests
of this nucleic acid in the 1940's revealed four residues differing by the presence
of different nucleotides, and further demonstrated that the concentrations of
A = T, and the concentration of C = G. At the time, it was known that chromosomes
contained both nucleic acid and proteins. And, given that proteins were built
with an alphabet of 20 amino acids and nucleic acids were only built with an
alphabet of 4 nucleotides, most scientists hypothesized that the complexity
of life was probably encoded by the more complex protein fraction of chromosomes,
rather than the less complex nucleic acid fraction. But rather than just hypothesize
and theorize, scientists got down to experimentally testing these ideas. Avery,
McCarty, and MacLeod (1944) conducted elegant experiments involving Streptococcus
pneumoniae bacteria. Like most bacteria, these organisms can exchange or
transfer genetic infomration; and the recipient then expresses traits of the
donor. These changes are heritable, meaning that the recipient passes this new
ability on to daughter cells when it divides. Avery, McCarty, and MacLeod (1944)
demonstrated that heritable change only takes place when DNA is absorbed by
recipient cells, not when RNA or protein is present. In 1952, Hershey and Chase
confirmed this pattern for viruses, too; demonstrating that viruses transfer
DNA to the host cell they infect, not proteins. This transferred DNA is what
orchestrates the production of new viruses in the host; it is the genetic material.
Since 1944, scientists around the world had been racing to determine the structure
of DNA. In the U.S.A., Linus Pauling proposed a triple heix structure. In England,
two labs were hot on the trail. Maurice Wilkins and his associates Rosalind
Franklin and R. G. Gosling were at King's College in London; James Watson and
Francis Crick were at the cavendish Lab at Cambridge. Using experimental results
from Franklin's work, Watson and Crick proposed the correct double-helix structure.
In April of 1953, their short paper and several others describe the
structure of DNA.
Like all good research, this work
answered one important question and opened up many others for analysis. Most
immediately, HOW does this molecule work to influence the characteristics of
an organism? The fact that DNA was only in the nucleus but proteins were made
in the cytoplasm, and that RNA was found in both places, eventually led to the
discovery of the structure and function of m-, r-, and t-RNA. In the early 19060's,
Marshall Nirenberg
and coworkers invented an ingenious technique for determining how the sequence
of nitrogenous bases in m-RNA coded for a sequence of amino acids in the protein
product. through these experiments, scientists discovered the genetic code.
We'll stop our overview here for now!
 A.
DNA and RNA Structure
A.
DNA and RNA Structure
DNA (deoxyribonucleic acid) and RNA
(ribonucleic acid) are nucleic acids - polymers consisting of a linear
sequence of linked nucleotide monomers. We will describe the structure of the
monomers first, and then describe how they are linked into linear polymers.
Finally, we will describe the double-stranded structure of ds-DNA.
1. The monomers
are "nucleotides"
three components:
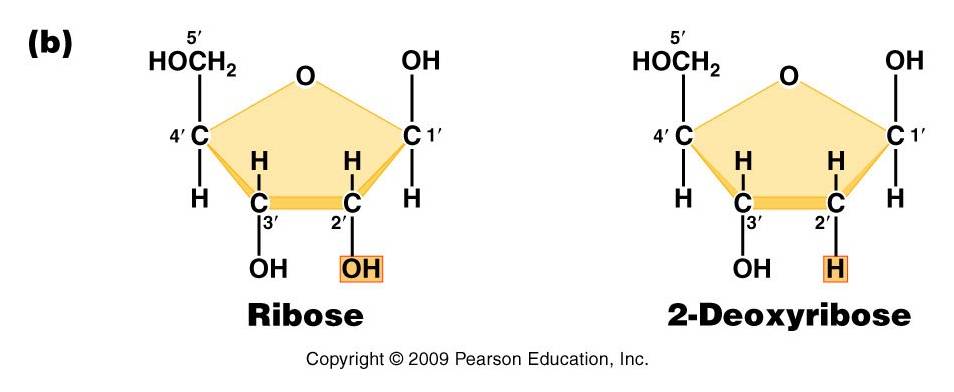
 -
Pentose (5 carbon) sugar: either ribose (RNA) or deoxyribose
(DNA). The carbons are numbered clockwise. The difference between the sugars
is that ribose has an -OH group on the 2' carbon, whereas deoxyriboes has
only 2 H groups and thus is "deoxygenated" relative to ribose. BOTH
sugars have an -OH group on the 3' carbon, which will be involved in binding.
The 5' carbon is a sidegroup off the ring.
-
Pentose (5 carbon) sugar: either ribose (RNA) or deoxyribose
(DNA). The carbons are numbered clockwise. The difference between the sugars
is that ribose has an -OH group on the 2' carbon, whereas deoxyriboes has
only 2 H groups and thus is "deoxygenated" relative to ribose. BOTH
sugars have an -OH group on the 3' carbon, which will be involved in binding.
The 5' carbon is a sidegroup off the ring.
-
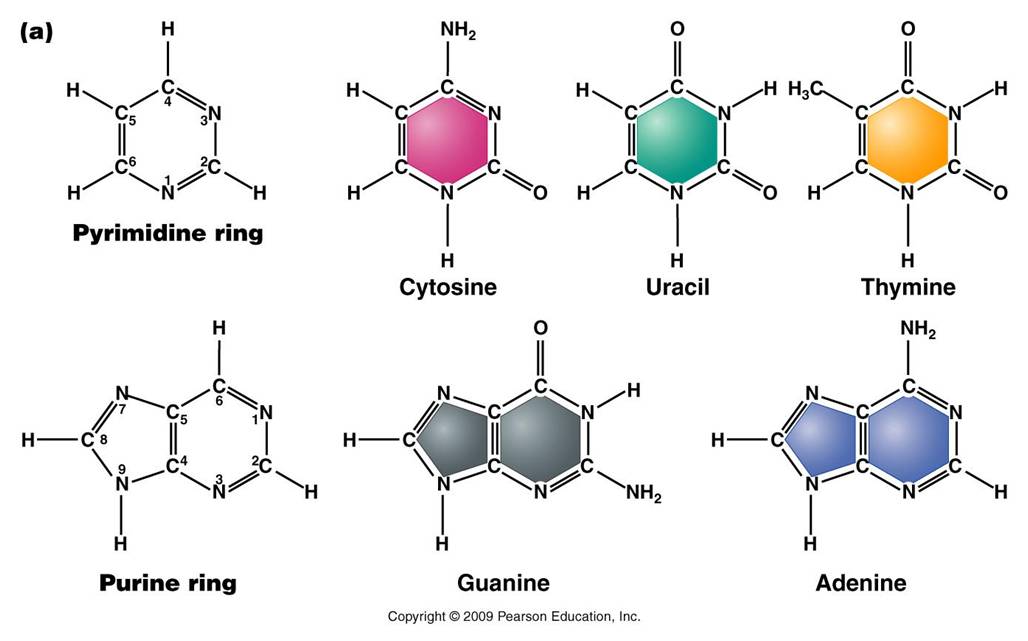
Nitrogenous Base: each nucleotide has a single nitrogenous
base attached to the 1' carbon of the sugar. This nitrogenous base may be
a double-ringed structure (purine) or a single ringed (pyrimidine) structure.
The purines are adenine (A) and guanine (G). The pyrimidines are thymine (T),
cytosine (C), and uracil (U). DNA nucleotides may carry A, G, C, or T. RNA
nucleotides carry either A, G, C, or U.
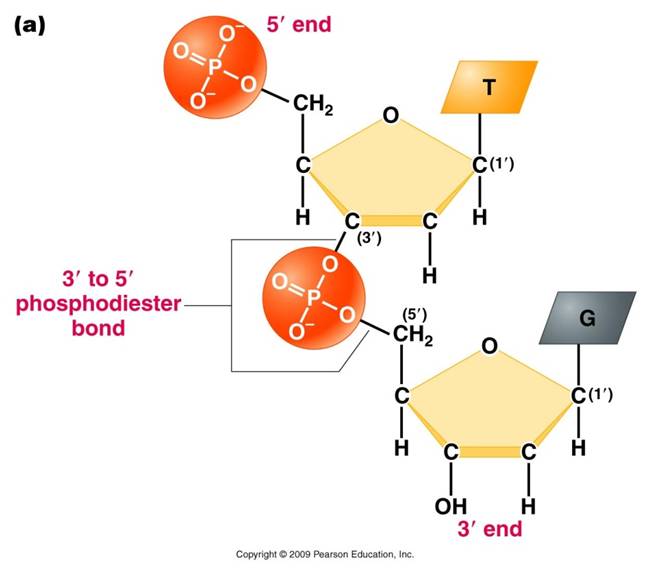 -
The third component of a nucleotide is a phosphate group,
which is attached to the 5' carbon of the sugar. When a nucleotide is incorporated
into a chain, it has a single phosphate group. However, nucleotides can occur
that have two or three phosphate groups (dinucleotides and trinucleotides).
ADP and ATP are important examples of these types of molecules. In fact, the
precursors of incorporated nucleotides are trinucleotides. When two phosphates
are cleaved, energy is released that can be used to add the remaining monophosphate
nucleotide to the nucleic acid chain.
-
The third component of a nucleotide is a phosphate group,
which is attached to the 5' carbon of the sugar. When a nucleotide is incorporated
into a chain, it has a single phosphate group. However, nucleotides can occur
that have two or three phosphate groups (dinucleotides and trinucleotides).
ADP and ATP are important examples of these types of molecules. In fact, the
precursors of incorporated nucleotides are trinucleotides. When two phosphates
are cleaved, energy is released that can be used to add the remaining monophosphate
nucleotide to the nucleic acid chain.
2. Polymerization
is by 'dehydration synthesis'
As with all other classes of biologically
important polymers, monomers are linked into polymers by dehydration synthesis.
In nucleic acid formation, this involves binding the phosphate group of one
nucleotide to the -OH group on the 3' carbon of the existing chain. For the
purposes of seeing how this reaction works, we can envision an H+ on one of
the negatively charged oxygens of the phosphate group. Then, a molceule of water
can be removed from these two -OH groups, leaving an oxygen binding the sugar
of one nucleotide to the phosphate of the next.
This creates a 'dinucleotide'. It
has a polarity/directionality; it is different at its ends. At one end, the
reactive group is the phosophate on the 5' carbon. This is called the 5' end
of the chain. At the other end, the reactive group is the free -OH on the 3'
carbon; this is the 3' end of the chain. So, a nucleic acid strand has a 5'
- 3' polarity.
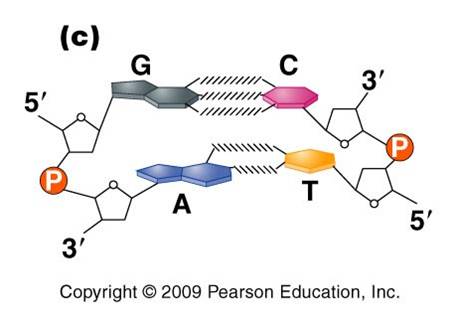 3.
Most DNA exists as a 'double helix' (ds-DNA) containing two linear nucleic acid
chains.
3.
Most DNA exists as a 'double helix' (ds-DNA) containing two linear nucleic acid
chains.
a. the
nitrogenous bases on the two strands are 'complementary' to each other,
and form weak hydrogen bonds between them. A always pairs with T, and C always
pairs with G. As such, there is always a double-ringed purine pairing with
a single-ringed pyrimidine, and the width of the double-helix is constant
over its entire length.
b. the
two strands (helices) are anti-parallel:
they are arranged with opposite polarity. One strands points 5' - 3', while
the other points 3' - 5'. The direction of the pentose sugars and the type
of reactive group at the ends of the chains show this relationship.
4. RNA performs
a wide variety of functions in living cells:
a. m-RNA
(for "messenger") is the copy of
a gene. It is the
sequence of nitrogenous bases in m-RNA that is actually read by the ribosome
to determine the structure of a protein.
b. r-RNA
(for "ribosomal")
is made the same way, as a copy of DNA. However, it is not carrying the recipe
for a protein; rather, it is functional as RNA. It is placed IN the Ribosome,
and it helps to ‘read’ the m-RNA.
c. t-RNA
(for "transfer")
is also made as a copy of DNA, but it is also functional as an RNA molecule.
Its function is to bind to a specific amino acid and incorporate it into the
amino acid sequence as instructed by the m-RNA and ribosome.
d. mi-RNA
(micro-RNA) and si-RNA (small interfering RNA)
bind to m-RNA and splice it; inhibiting the synthesis of its protein. This
is a regulatory function.
e.
sn-RNA (small nuclear RNA) are short sequences that process
initial m-RNA products, and also regulate the production of r-RNA, maintain
telomeres, and regulate the action of transcription factors. Regulatory functions.
 B.
Chromosome Structure
B.
Chromosome Structure
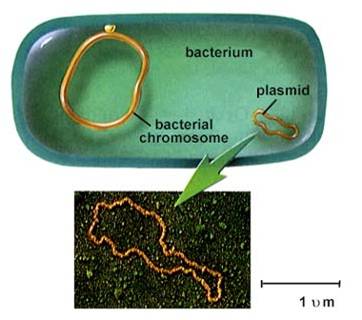
1. Prokaryotes
- usually one circular chromosome, tethered to the membrane, with some associated,
non-histone proteins.
2. Eukaryotes
– usually many linear chromosomes,
highly condensed with histone proteins into several levels of structure.
Level 1:
ds-DNA is wrapped around histone proteins, creating the “beads on a
string’ level of organization.
Level 2: string is coiled, 6
nucleosomes/turn (solenoid)
Level 3: the coil is ‘supercoiled’
Level 4: the supercoil is folded
into a fully condensed metaphase chromosome
 To
read a gene, the chromosome must be diffuse (uncondensed) in that region. Even
when condensed, these ‘euchromatic’ coding regions are less condensed
and more lightly staining than non-coding regions.
To
read a gene, the chromosome must be diffuse (uncondensed) in that region. Even
when condensed, these ‘euchromatic’ coding regions are less condensed
and more lightly staining than non-coding regions.
DNA that has few genes can remain
condensed and closed (heterochromatic), and appears as dark bands on condensed
chromosomes.
Study Questions:
1) Diagram the
parts of an RNA nucleotide.
2) Show
how two nucleotides are linked together by dehydration synthesis reactions.
3) Why does the
purine - pyrimidine structure relate to the complementary nature of double-stranded
DNA?
4) Draw
a DNA double helix, showing three base pairs and the antiparallel nature of
the helices.
5) Describe
the higher levels of eukaryotic chromosome structure, including the terms nucleosome
and solenoid.
6) What are
two differences between euchromatin and hetochromatin?
 Much of the energy harvested by a cell is used to make proteins. However, in order to understand protein synthesis, we must first describe the structure of DNA. DNA is the 'recipe' for proteins, so we need to take a little detour on our jounrey of how a cell works to describe the structure of DNA and chromosomes. DNA
is the genetic material in all forms of life (eubacteria, archaea, protists,
plants, fungi, and animals). Those quasi-living viruses vary in their genetic
material. Some have double-stranded DNA (ds-DNA) like living systems, while
others have ss-DNA, ss-RNA, and ds-RNA. RNA performs a wide array of functions
in living systems. Many of these functions have only been discovered in the
last few years.
Much of the energy harvested by a cell is used to make proteins. However, in order to understand protein synthesis, we must first describe the structure of DNA. DNA is the 'recipe' for proteins, so we need to take a little detour on our jounrey of how a cell works to describe the structure of DNA and chromosomes. DNA
is the genetic material in all forms of life (eubacteria, archaea, protists,
plants, fungi, and animals). Those quasi-living viruses vary in their genetic
material. Some have double-stranded DNA (ds-DNA) like living systems, while
others have ss-DNA, ss-RNA, and ds-RNA. RNA performs a wide array of functions
in living systems. Many of these functions have only been discovered in the
last few years.  A.
DNA and RNA Structure
A.
DNA and RNA Structure -
Pentose (5 carbon) sugar: either ribose (RNA) or deoxyribose
(DNA). The carbons are numbered clockwise. The difference between the sugars
is that ribose has an -OH group on the 2' carbon, whereas deoxyriboes has
only 2 H groups and thus is "deoxygenated" relative to ribose. BOTH
sugars have an -OH group on the 3' carbon, which will be involved in binding.
The 5' carbon is a sidegroup off the ring.
-
Pentose (5 carbon) sugar: either ribose (RNA) or deoxyribose
(DNA). The carbons are numbered clockwise. The difference between the sugars
is that ribose has an -OH group on the 2' carbon, whereas deoxyriboes has
only 2 H groups and thus is "deoxygenated" relative to ribose. BOTH
sugars have an -OH group on the 3' carbon, which will be involved in binding.
The 5' carbon is a sidegroup off the ring. -
The third component of a nucleotide is a phosphate group,
which is attached to the 5' carbon of the sugar. When a nucleotide is incorporated
into a chain, it has a single phosphate group. However, nucleotides can occur
that have two or three phosphate groups (dinucleotides and trinucleotides).
ADP and ATP are important examples of these types of molecules. In fact, the
precursors of incorporated nucleotides are trinucleotides. When two phosphates
are cleaved, energy is released that can be used to add the remaining monophosphate
nucleotide to the nucleic acid chain.
-
The third component of a nucleotide is a phosphate group,
which is attached to the 5' carbon of the sugar. When a nucleotide is incorporated
into a chain, it has a single phosphate group. However, nucleotides can occur
that have two or three phosphate groups (dinucleotides and trinucleotides).
ADP and ATP are important examples of these types of molecules. In fact, the
precursors of incorporated nucleotides are trinucleotides. When two phosphates
are cleaved, energy is released that can be used to add the remaining monophosphate
nucleotide to the nucleic acid chain. 3.
Most DNA exists as a 'double helix' (ds-DNA) containing two linear nucleic acid
chains.
3.
Most DNA exists as a 'double helix' (ds-DNA) containing two linear nucleic acid
chains. B.
Chromosome Structure
B.
Chromosome Structure To
read a gene, the chromosome must be diffuse (uncondensed) in that region. Even
when condensed, these ‘euchromatic’ coding regions are less condensed
and more lightly staining than non-coding regions.
To
read a gene, the chromosome must be diffuse (uncondensed) in that region. Even
when condensed, these ‘euchromatic’ coding regions are less condensed
and more lightly staining than non-coding regions.