Waterworld
Goals:
1. Understand how liquid water has changed the composition of the atmosphere.
2. Review the fundamentals of atoms and their interactions (bonds)
3. Understand how the molecular structure of water contributes to its properties.
4. Understand why these properties are so important, and make water the 'solute of life'.
5. Understand how life makes earth even more differnt from other planets.
I. The Earth and its Neighbors
A. Size and Temperature
Why is Earth the only living planet? One philosophical approach that is used in science (and other disciplines) to answer questions is "the comparative method". If you want to understand why something "is the way it is", compare it to other things... then you developed a list of other correlated differences; one of these might cause the characteristic of interest. For example, our question is this: "Why does life exist only on Earth"? Well, let's compare Earth to other planets similar to it, like Venus and Mars. Maybe one of these other differences--or a combination of them--is why Earth is the only planet with life.
Mars is the fourth planet from the sun (Earth is third) and second smallest planet in the solar system; only Mercury is smaller. It is likely that the growth of Jupiter prohibited planetissimals from accreting onto Mars or condensing into a larger separate planet—they continue to orbit as the unconsolidated ‘asteroid belt’ between the orbits of Mars and Jupiter. Mars is about ½ the size of earth in radius and equatorial circumference. Its “day” is about 24 hrs, but it takes 1.88 earth years to orbit the sun. Although Earth temperatures range between -88 and 58oC (136oF), Mars ranges from -153 to 20oC (68oF). All surface water is bound up in a polar ice cap, although erosional landscapes suggest that liquid water did flow over the surface in the past. Venus is the second planet from the sun and is only slightly smaller (95%) than the Earth. It spins slowly (and opposite to Earth), taking 117 earth days to complete one rotation, and only spins a bit more than once per year (225 Earth days). Surface temperatures reach 462oC (~900oF). Venus is constantly clothed in clouds because most compounds vaporize at these temps; the clouds force a “runaway greenhouse effect” that traps heat. All of the scant water exists as water vapor. These temperature differences contribute to the first major difference between Earth and these planets is that Earth is covered (71%) by liquid water.
B. Atmospheric Composition
All three planets (and Mercury) are “rocky”, as opposed to the gas giants in the outer solar system. Mars and Venus have very similar atmospheres, composed largely of CO2 with a small amount of N2. In contrast, Earth has little CO2. The reduction in this quantity makes the proportional representation of N2 increase dramatically. And of course, there is O2 in the Earth’s atmosphere, which is all but absent in the atmospheres of the other planets.
|
Earth |
Venus |
Mars |
CO2 |
0.035% |
96% |
95% |
N2 |
77% |
3.5% |
2.7% |
H2O |
1% |
0.01% |
0.007% |
Ar |
0.93% |
0.007% |
1.6% |
O2 |
21% |
trace |
trace |
II. Why these Differences?
A. The Effects of Liquid Water
The Earth differs from Mars and Venus in having life, and in being covered by water. Perhaps having water is, in part, why Earth has life. (This is the power of the comparative method.) Water has a number of properties that are probably necessary for life to exist. First and foremost, it is a solvent that dissolves many other materials: this allows their separated components to interact with one another in new combinations, in new chemical reactions. Why does water hav this property? Our second philosophical approach used in science is "reductionism". We have found that we can answer many questions about how and why something "is as it is" by 'breaking it apart' - by seeing what it is made of - by 'reducing' it to its parts. How does a human body work? Well, let's cut it open and see what is inside - let's describe the 'subsystems' (like the circulatory system and respiratory system) and maybe we will gain an appreciation of how the entire system works. Water is matter. Like all matter, it is composed of atoms. Perhaps we can understand the properties of water if we understand how the atoms in it are arranged. Before we do this, however, we need to have a quick review of atoms and molecules.
- Atoms and Bonds tangent
1. conventional matter - defined as a substance that has mass and occupies space - consists of atoms.
2. there are 94 different types of naturally occurring atoms - defined by differences in the number of protons in the nucleus. These are the naturally occurring elements which have different properties (mass and charge, for instance) as a consequence of their different number of protons. Atoms are also defined as the smallest unit of a pure substance (one element) that retains the properties of that substance and cannot be subdivided further by chemical means.
3. compounds are substances composed of two or more types of elements in a fixed ratio, with a particular structure/spatial arrangement maintained by chemical bonds (ionic or covalent). So, NaCl (table salt) is an ionic compound. H2O (water) is a covalent compound.
4. molecules are substances of two or more atoms bound together by covalent bonds. So, it is inappropriate to refer to a 'molecule' of NaCl (which is ionic). Two atoms of hydrogen bound together by a covalent bond is a molecule of hydrogen. It is not a compound, as it only contains one type of element. For compounds consisting of covalently bound atoms, a molecule is the smallest unit of the compound that maintains the properties of that compound. So, we can have one molecule of water, which is also a compound.
- Atoms
We will use a very simplistic 'Bohr' model of atomic structure, emphasizing the particulate nature of matter (rather than the wave nature) and excluding a consideration of quarks. For a more detailed account, feel free to see this link from the University of Oregon and Wikipedia.
1. atoms have a nucleus containing protons (mass ~ 1 atomic mass unit; elementary charge = +1 ) and neutrons (mass ~ 1 atomic mass unit; elementary charge = 0). The number of protons defines the type of atom - the element - and it's atomic number. The mass of an atom is largely determined by the mass of protons and neutrons. Although all atoms of an element have the same number of protons, the number of neutrons can vary. These atoms with variable numbers of neutrons are called isotopes. Those with an excess number of neutrons are less stable and will loose them over time. These are radioisotopes, and they emit energy when they loose a neutron. They loose them at a constant rate, so you can date how old they are by how many have changed to the more stable state. Atoms with unequal numbers of protons and electrons have a net charge and are called ions.
2. the nucleus is surrounded by a cloud of electrons (mass ~ 0; elementary charge = -1), represented as shells and orbitals: Shells 1, 2, 3 have 1, 4, 9 orbitals, 'containing' a maximum of 2, 8, 18 electrons, respectively. The orbitals are 1000's of times the width of the nucleus, so an atom (and hence, all matter) is mostly space. For comparison, if you envision the nucleus of a carbon atom as a basketball (about 12 inches in diameter), the outermost electrons would be orbiting 5 miles away.
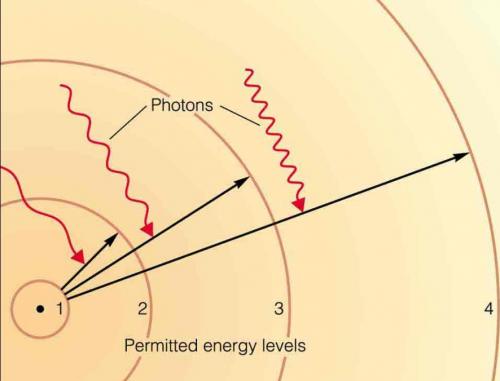 3. the distance between the orbital and the nucleus correlates with the energy of the electron (in terms of a wave function).
3. the distance between the orbital and the nucleus correlates with the energy of the electron (in terms of a wave function).
4. as electrons gain energy, they 'orbit' farther from nucleus. Without an input of energy, an atom will lose energy and approach it's lowest energy state, occupying the closest 'unoccupied' orbital available.
5. changing orbitals - and energy states - is a discrete process. Electrons must gain or lose discrete amounts of energy (quanta) to change position to another orbital.
6. Binding properties are largely governed by the number of 'valence' electrons in the outermost orbital/shell. They achieve greater stability when the outermost orbitals/shells are full. They achieve this stability by losing, gaining, or sharing electrons with other atoms. For the first three shells, the valence electrons are 2, 8, and 8... hence the 'octet rule'.
- Bonds
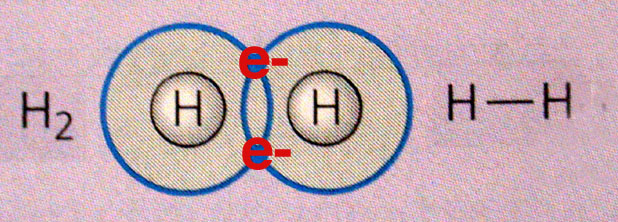 1. Covalent Bonds
1. Covalent Bonds
Atoms share electons in pairs, each contributing one electron to the shared pair: H2, H2O, etc. These are the primary bonds in biologically important molecules - they can be non-polar (shared evenly) like H2 - or polar (shared unevenly...creating a charge difference across the molecule - as in water, where the shared electrons are held more tightly by the larger oxygen nucleus, pulling the electron cloud off the hydrogen nucleus, revealing some of its positive charge).
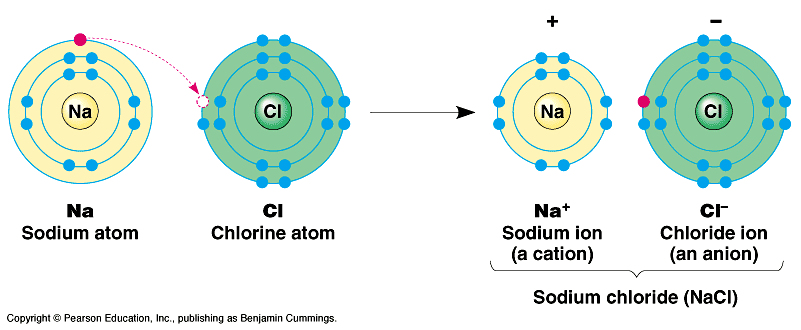
2. Ions and Ionic Bonds
Electrons can be transferred completely from one atom to another. This creates charged particles (ions) which may then be attracted to one another based on their opposite charge. (NaCl)
3. Hydrogen Bonds
These are weak ionic bonds, weak forces of attraction between partially charged regions of different molecules. These partial charges arise because, in polar covalent bonds, the unequal sharing of electrons gives one atom a slight negative charge and the other a slight positive charge. This is common when hydrogen is invovled because, as the smallest atom, all others elements will attract the shared electrons more forecefully.
1. Water’s molecular structure
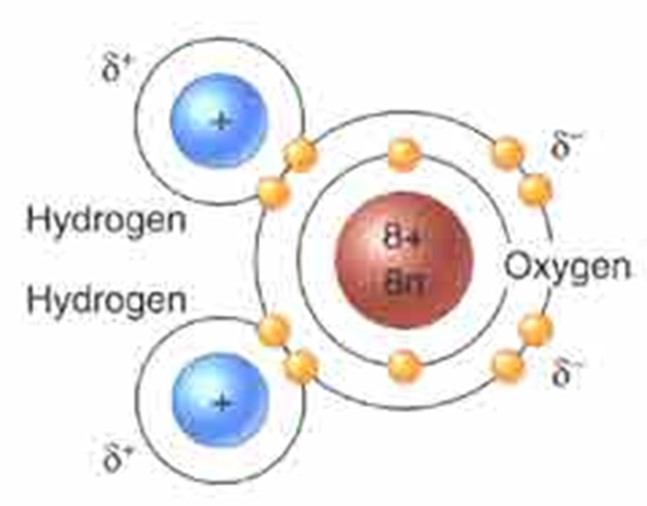 Water has numerous properties that affect Earth (and life). Water is composed of one oxygen atom, with 8 protons, and 8 neutrons in the nucleus, orbited by 8 electrons. There are 2 electrons orbiting in the first “shell” and 6 in the second. The second shell can hold 8, and atoms are most stable if the outer shell is full ('octet rule'). So, oxygen will interact with other atoms and share pairs of electrons to “fill” its outer shell. This sharing of electrons between atoms is called a “covalent bond”. One oxygen atom will form single covalent bonds with two hydrogen atoms, each that has only one electron in its first shell. By sharing pairs of electrons, each atom fills its outer shell and is stable.
Water has numerous properties that affect Earth (and life). Water is composed of one oxygen atom, with 8 protons, and 8 neutrons in the nucleus, orbited by 8 electrons. There are 2 electrons orbiting in the first “shell” and 6 in the second. The second shell can hold 8, and atoms are most stable if the outer shell is full ('octet rule'). So, oxygen will interact with other atoms and share pairs of electrons to “fill” its outer shell. This sharing of electrons between atoms is called a “covalent bond”. One oxygen atom will form single covalent bonds with two hydrogen atoms, each that has only one electron in its first shell. By sharing pairs of electrons, each atom fills its outer shell and is stable.
However, since the nucleus of the oxygen atom (with 8 positively charged protons) attracts the negative electrons more than the one proton in each hydrogen atom, the electron pairs are not shared evenly; they are held more closely to the oxygen, so it is slightly negative… the hydrogens have their negativity pulled away from their proton, revealing a part of its positive charge. So, there is a ‘polarity’ to a water molecule – a positively charged end and a negatively charged end. This is a critical property: WATER IS POLAR. Because water is polar, it forms hydrogen bonds with itself. If the free energy in the system is high, then the molecules will be too exited to bond with one another and the sate will be water vapor. If the free energy is low, then the molecules will all bind, forming a lattice of molecules in solid ice. In between, (0 - 100oC) bonds break and reform and the molecules shift connections but are rarely completely free--existing as liquid water.
2. Water is called the “universal solvent” - Charged compounds (polar or ionic) dissolve in water
Many materials have charges, sometimes for the same reason that water does. Sugar molecules are partially charged, like water, and so sugar molecules can become separated from themselves in water, binding to water instead of one another.
Some materials (atom or molecule) have an unequal number of protons and electrons, and thus have a “complete” charge of -1 or +1, depending on whether the surplus is an electron or proton. They can have larger charges (like +3), too. Since they are charged, they can also attract and interact with water molecules and thus become separated (dissolve).
Table salt (sodium chloride) is a typical example of an “ionic compound”, composed of Na+ and Cl- atoms that are attracted to one another because of their opposite charges. Typically, these oppositely charged ions attract one another in a lattice/crystal. But placed in water, the ions become separated and attracted to water…. Dissolving.
3. Water dissociates into ions
Think of the electrons shared between the large oxygen nucleus and each small hydrogen nucleus (1 proton) as a “bed sheet” of electronegativity. When the larger oxygen nucleus rolls itself up in the sheet, it pulls the sheet partial off the hydrogen nucleus, revealing a portion of the proton’s positive charge. Sometimes, the oxygen pulls the sheet completely away from the hydrogen; now they aren’t sharing the electron-pair at all; the hydrogen nucleus is ‘naked’ – just 1 proton – existing as an independent H+ ion. The oxygen (and the other hydrogen) now have an extra electron and are a negatively charged hydroxide ion. The water molecule has “dissociated” into H+ and OH- ions. Now, of course, these oppositely charged ions are attracted to one another and they will re-associate. Water molecules are constantly splitting and rejoining in solution. In pure water, 1 in 10,000,000 (10 million) water molecules is dissociated at any one time. This equals a concentration of 1 x 10-7, and the negative of the exponent that describes the concentration of free H+ ions is an important characteristic of a liquid… it is called the pH (sort of the “percent Hydrogen”). In this case, pure water has a pH = 7.0. Hydrogen ions are very reactive. So, fluids with high concentrations of free H ions (low pH) are very reactive. Consider what happens between Cl (with 17 P and 17 e) and Hydrogen (1 p, 1 e). They share a pair of electrons and fill their outermost shells, making HCl (Hydrochloric acid). But, since the Cl is SO MUCH BIGGER (twice the pull of oxygen’s 8 protons), it rips the electron pair away from Hydrogen more frequently. In fact, the concentration of dissociated HCl molecules in solution, and thus the concentration of free H+ ions, is not 1 in 10 million like with water, it is 1 in 100! This is represented in exponential notation as 1 x 10-2, and we say that HCl has a pH = 2.0.
4. Water weathers rock, putting ions into solution
This is important because ROCKS can dissolve in water. Water also erodes rock, breaking pieces off mechanically and putting them in suspension and carrying them to other places. These physical and chemical processes of rock breakdown are called “weathering”. Water gets rocks moving and puts ions in solution where they can react with other chemicals.
One way that water dissolves rock is by “cation displacement”.
The most abundant minerals in the Earth’s crust are feldspars – they make up 60% of the Earth’s crust. Chemically, they are KAlSi3O8, NaAlSi3O8, and CaAlSi3O8. K+, Na+, and Ca+2 are positively changed ions. In the presence of water, the dissociated H+ ions ‘displace’ these other cations (positive ions), which are soluble in water because of their charge, leaving the silicate behind.
5. Carbon dioxide reacts with water to form carbonic acid (H2CO3).
CO2 is soluble in water, and it combines with water to form carbonic acid. Acids dissociate, giving up their H+ ions, as we know. Carbonic acids in the soil are much more important in dissolving rock than water, as they have a pH of 5.6 (more acidic – a higher tendancy to give up H+). Carbonic acid dissociates, forming bicarbonate and carbonate. The carbonate reacts with Ca+2 in solution, forming “calcium carbonate” which settles out of the water as limestone, or precipitates when the water evaporates. This is the “abiogenic” (without life) process that forms limestone. Most limestone forms biogenically, though, as we will see.
B. The Earth’s crust is recycled by tectonic activity
Mars is too small to generate the internal heat necessary for plate tectonics. Plate tectonics recirculates the Earth’s crust, taking some carbon from the atmosphere, that has been deposited in sediments, deep into the mantle. Venus is large enough to generate tectonic activity, but the crust is so hot that it “melts” and heals rather than forming plates that can be forced under one another, as on Earth. So it melts and crusts up, but doesn’t form plates. So, as a consequence of liquid water on Earth, CO2 in the atmosphere is dissolved, where it reacts with water and calcium and precipitates as limestone, which can be transported by plate tectonics deep into the Earth for long-term storage. Volcanoes return carbon to the atmosphere as carbon dioxide, but there is a net transfer to the lithosphere.
C. The Effects of Life
 1. Biogenic Limestone Formation
1. Biogenic Limestone Formation
Many marine organisms, including some types of photosynthetic algae (Coccolithophores), Molluscs (clams, oysters, mussels, snails), and corals absorb calcium and carbonate and form calcium carbonate to make their shells. When they die, these shells fall to the bottom of the ocean, accumulate, and under pressure are turned to limestone. Under enough pressure and heat, they metamorphose into marble. 10% of the Earth’s rock is limestone, and most of this was produced organically (biologically). As CO2 is converted to limestone, more CO2 dissolves in water. So, life sucks CO2 out of the air and into the oceans, converting it to limestone.
2. Photosynthesis
 As we will learn in more detail later, photosynthesis typically consists of two major reactions: the “light dependent” and “light independent” reactions. In the light dependent reactions, organisms transform radiant energy of sunlight, carried by photons, into chemical energy in chemical bonds, carried by electrons. So, in sunlight, photosynthetic organisms trap convert light energy into chemical bonds…typically by binding a phosphate (PO4) to adenosine diphosphate (ADP) to make ATP (adenosine triphosphate). To get the electrons that carry this radiant energy, the organisms split water molecules… the freed oxygen atoms from 2 water molecules unite, to form a molecule of oxygen gas (O2). So, photosynthetic organisms split water to harvest electrons, and oxygen gas is released as a waste product. All of the oxygen gas in our atmosphere (21%!!!) was produced by this process.
As we will learn in more detail later, photosynthesis typically consists of two major reactions: the “light dependent” and “light independent” reactions. In the light dependent reactions, organisms transform radiant energy of sunlight, carried by photons, into chemical energy in chemical bonds, carried by electrons. So, in sunlight, photosynthetic organisms trap convert light energy into chemical bonds…typically by binding a phosphate (PO4) to adenosine diphosphate (ADP) to make ATP (adenosine triphosphate). To get the electrons that carry this radiant energy, the organisms split water molecules… the freed oxygen atoms from 2 water molecules unite, to form a molecule of oxygen gas (O2). So, photosynthetic organisms split water to harvest electrons, and oxygen gas is released as a waste product. All of the oxygen gas in our atmosphere (21%!!!) was produced by this process.
In the light independent reactions, the chemical energy now present in the new bonds in ATP is converted into a more stable bond between carbon atoms. So, ATP is “broken” and the energy released is used to make bonds between carbon atoms. Where does the carbon come from? CO2 in the air.
So, photosynthetic organisms use the ATP made in the light dependent reactions to link 6 CO2 molecules together, making a glucose molecule that has six carbons. Photosynthesis contributes to the two major differences between the Earth’s atmosphere and those of Mars and Venus: it produces the O2 and sucks CO2 out of the atmosphere and makes glucose.
3. The Biosphere and the Carbon Cycle
Today, these effects can be seen in the annual cycles of CO2 and oxygen levels in the atmosphere. Most of the land mass on our planet is on the northern hemisphere. so, from March-September, photosynthesis exceeds respiration at a planetary scale and O2 increases while CO2 drops. From October to February, even though this is the southern hemisphere’s summer, global photosynthesis < respiration and CO2 levels rise and O2 levels drop. The earth breathes.
And, of course, as we cut forests, we reduce the amount of CO2 that is absorbed by plants each year, and reduce the amount of O2 produced. So, CO2 levels oscillate and rise, while O2 levels oscillate and drop. CO2 is increasing, as well, as a consequence of burning fossil fuels—returning the CO2 absorbed by plants 350 million years ago back to air.
Conclusion: The Earth is a LIVING PLANET. Life doesn’t just live on Earth. It has transformed Earth, and it continues to maintain these odd conditions. Recent human activity is changing these relationships that have evolved over billions of years.
Things to Know (between your ears, not between the pages of your notebook!):
1. Know the structure of an atom, the characteristics and location of protons, neutrons, and electrons, and how elements, isotopes, and ions differ.
2. Describe the three ways atoms interact, with an example for each.
3. Know the differnece between polar and non-polar bonds and molecules, and why this difference occurs.
4. Know what pH measures, and what it means, quantitatively, that pure water has a pH = 7.
5. Know how water dissolves rock, and how carbon dioxide dissolves in water and is converted to limestone.
Study Questions (Put your notes aside and see if you can answer these COMPLETELY):
1. Draw a water molecule, showing the atoms and electrons. Explain how this structure causes water to be the "universal solvent".
2. Water has a high 'specific heat'. Why, and why is this important to life?
3. Outline the process of photosynthesis, and state the two ways it affects the composition of the atmosphere.
4. How and why do CO2 and O2 levels vary over the course of a year?
5. How and why is CO2 increasing and O2 decreasing from decade to decade? Describe two reasons.
 3. the distance between the orbital and the nucleus correlates with the energy of the electron (in terms of a wave function).
3. the distance between the orbital and the nucleus correlates with the energy of the electron (in terms of a wave function).  1. Covalent Bonds
1. Covalent Bonds
 Water has numerous properties that affect Earth (and life). Water is composed of one oxygen atom, with 8 protons, and 8 neutrons in the nucleus, orbited by 8 electrons. There are 2 electrons orbiting in the first “shell” and 6 in the second. The second shell can hold 8, and atoms are most stable if the outer shell is full ('octet rule'). So, oxygen will interact with other atoms and share pairs of electrons to “fill” its outer shell. This sharing of electrons between atoms is called a “covalent bond”. One oxygen atom will form single covalent bonds with two hydrogen atoms, each that has only one electron in its first shell. By sharing pairs of electrons, each atom fills its outer shell and is stable.
Water has numerous properties that affect Earth (and life). Water is composed of one oxygen atom, with 8 protons, and 8 neutrons in the nucleus, orbited by 8 electrons. There are 2 electrons orbiting in the first “shell” and 6 in the second. The second shell can hold 8, and atoms are most stable if the outer shell is full ('octet rule'). So, oxygen will interact with other atoms and share pairs of electrons to “fill” its outer shell. This sharing of electrons between atoms is called a “covalent bond”. One oxygen atom will form single covalent bonds with two hydrogen atoms, each that has only one electron in its first shell. By sharing pairs of electrons, each atom fills its outer shell and is stable.
 As we will learn in more detail later, photosynthesis typically consists of two major reactions: the “light dependent” and “light independent” reactions. In the light dependent reactions, organisms transform radiant energy of sunlight, carried by photons, into chemical energy in chemical bonds, carried by electrons. So, in sunlight, photosynthetic organisms trap convert light energy into chemical bonds…typically by binding a phosphate (PO4) to adenosine diphosphate (ADP) to make ATP (adenosine triphosphate). To get the electrons that carry this radiant energy, the organisms split water molecules… the freed oxygen atoms from 2 water molecules unite, to form a molecule of oxygen gas (O2). So, photosynthetic organisms split water to harvest electrons, and oxygen gas is released as a waste product. All of the oxygen gas in our atmosphere (21%!!!) was produced by this process.
As we will learn in more detail later, photosynthesis typically consists of two major reactions: the “light dependent” and “light independent” reactions. In the light dependent reactions, organisms transform radiant energy of sunlight, carried by photons, into chemical energy in chemical bonds, carried by electrons. So, in sunlight, photosynthetic organisms trap convert light energy into chemical bonds…typically by binding a phosphate (PO4) to adenosine diphosphate (ADP) to make ATP (adenosine triphosphate). To get the electrons that carry this radiant energy, the organisms split water molecules… the freed oxygen atoms from 2 water molecules unite, to form a molecule of oxygen gas (O2). So, photosynthetic organisms split water to harvest electrons, and oxygen gas is released as a waste product. All of the oxygen gas in our atmosphere (21%!!!) was produced by this process.